PLAQCLNZ EZ WIPES- zinc gluconate solution
PlaqClnz Ez Wipes by
Drug Labeling and Warnings
PlaqClnz Ez Wipes by is a Animal medication manufactured, distributed, or labeled by SmartHeatlh, Inc. (DBA SmartPractice), Addison Biological Laboratory, Inc., Jost Chemical Co., ADAMSON ANALYTICAL LABORATORIES INC, Alliance Analytical Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DOSAGE & ADMINISTRATION
- DESCRIPTION
- STORAGE AND HANDLING
-
PRINCIPAL DISPLAY PANEL
PlaqClnz®
Ez Wipes
Cleans
teeth, gums
& freshens
breath100 Soft Oral wipes for pets
Fresh Breath. Healthy Mouth. Happier pet.
1. Remove wipe and wrap around index finger
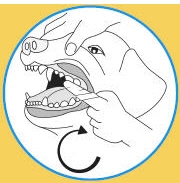
2. Lift pet's cheek and wipe along gum line on both sides of mouth.
For best results, use daily. Withhold food and water 30 minutes before and after application.INGREDIENTS: Deionized water, Zinc Gluconate, Taurine, Methylparaben, Propylparaben, F.D. & C. Blue #1
STORAGE: Store at room temperature and keep out of direct sunlight. Keep out of reach of children.
Item #24841
SmartPractice
Manufactured for SmartPractice®
Phoenix, AZ 85008 Made in USA
-
INGREDIENTS AND APPEARANCE
PLAQCLNZ EZ WIPES
zinc gluconate solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 69521-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Gluconate (UNII: U6WSN5SQ1Z) (Zinc Cation - UNII:13S1S8SF37) Zinc Gluconate 0.0165 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TAURINE (UNII: 1EQV5MLY3D) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BLUE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69521-005-05 12 in 1 BOX 1 100 in 1 CANISTER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 01/01/2016 Labeler - SmartHeatlh, Inc. (DBA SmartPractice) (061081477) Registrant - Addison Biological Laboratory, Inc. (832629203) Establishment Name Address ID/FEI Business Operations Addison Biological Laboratory, Inc. 832629203 manufacture Establishment Name Address ID/FEI Business Operations Jost Chemical Co. 147882294 api manufacture Establishment Name Address ID/FEI Business Operations ADAMSON ANALYTICAL LABORATORIES INC 944399906 analysis Establishment Name Address ID/FEI Business Operations Alliance Analytical Laboratories, Inc. 007588338 analysis
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.