BISACODYL tablet, coated
Bisacodyl by
Drug Labeling and Warnings
Bisacodyl by is a Otc medication manufactured, distributed, or labeled by KROGER COMPANY, TIME CAP LABORATORIES INC, TIME CAP LABS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
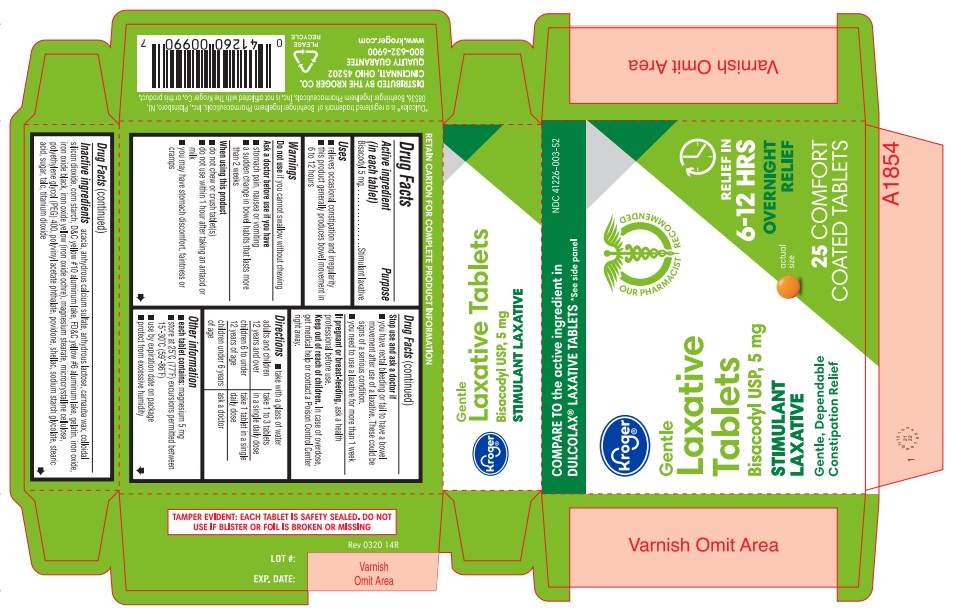
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients acacia, anhydrous calcium sulfate, anhydrous lactose, carnauba wax, colloidal silicon dioxide, corn starch, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, gelatin, iron oxide, iron oxide black, iron oxide yellow (iron oxide ochre), magnesium stearate, microcrystalline cellulose, polyethylene glycol (PEG) 400, polyvinyl acetate phthalate, povidone, shellac, sodium starch glycolate, stearic acid, sugar, talc, titanium dioxide
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BISACODYL
bisacodyl tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41226-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (DEACETYLBISACODYL - UNII:R09078E41Y) BISACODYL 5 mg Inactive Ingredients Ingredient Name Strength IRON OXIDES (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) TALC (UNII: 7SEV7J4R1U) STEARIC ACID (UNII: 4ELV7Z65AP) CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARNAUBA WAX (UNII: R12CBM0EIZ) POVIDONE (UNII: FZ989GH94E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) GELATIN (UNII: 2G86QN327L) FD&C YELLOW NO. 6 ALUMINUM LAKE (UNII: GYP6Z2JR6Q) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SUCROSE (UNII: C151H8M554) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ACETATE PHTHALATE (UNII: 58QVG85GW3) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) STARCH, CORN (UNII: O8232NY3SJ) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) ACACIA (UNII: 5C5403N26O) Product Characteristics Color orange Score no score Shape ROUND Size 5mm Flavor Imprint Code TCL003 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41226-003-52 25 in 1 BLISTER PACK; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 06/01/2020 Labeler - KROGER COMPANY (006999528) Registrant - TIME CAP LABORATORIES INC (037052099) Establishment Name Address ID/FEI Business Operations TIME CAP LABS 037052099 manufacture(41226-003)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.