ENMOTION HIGH FREQUENCY USE FOAM HAND SANITIZER- ethyl alcohol liquid
enMotion High Frequency Use Foam Hand Sanitizer by
Drug Labeling and Warnings
enMotion High Frequency Use Foam Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Georgia-Pacific Consumer Products, CYAN Labs S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Water (Aqua), Isopropyl Alcohol, PEG-12 Dimethicone, Glycerin, Hydrolyzed Jojoba Esters, Caprylic/Capric Triglyceride, Panthenol, Isopropyl Myristate, Aloe Barbadensis Leaf Juice, Zostera Marina Extract, Chamomilla recutita (Matricaria) Extract, Camelia Sinensis Leaf Extract, Avena Sativa (oat) Kernel Extract, Euterpe Oleracea Fruit Extract, Butylene Glycol, Sodium PCA, Sodium Lactate, Bisabolol, Hydrolyzed Glucosaminoglycans, Urea, Collagen Amino Acids, Lactic Acid, Phenoxyethanol, Citric Acid, Sodium Benzoate, Potassium Sorbate, Acetyl Hexapeptide-49
-
PRINCIPAL DISPLAY PANEL
enMotion
High Frequency Use Foam Hand Sanitizer
Fragrance Free, Dye Free
Espuma Desinfectante de Uso Frecuente
Sin Fragrancia, Sin Colorantes
Net Contents 2-950mL (32.1 FL OZ)
TOTAL: 1900mL (64.2 FL OZ)
Made in Mexico
enMotion is a trademark of Georgia-Pacific Consumer Products LP.
Contact Georgia-Pacific at 1-866-HELLOGP(1-866-435-5647)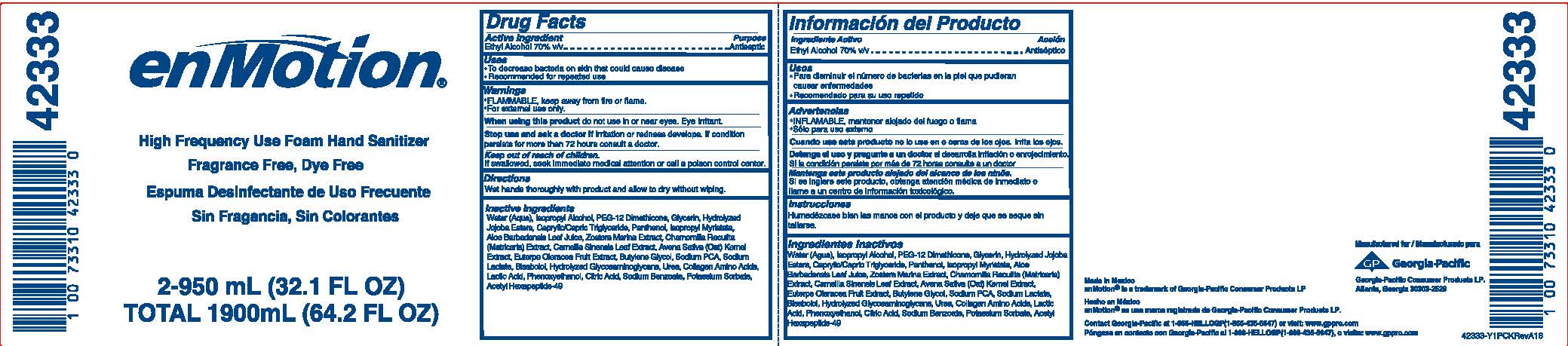
-
INGREDIENTS AND APPEARANCE
ENMOTION HIGH FREQUENCY USE FOAM HAND SANITIZER
ethyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54622-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM HYDROLYZED JOJOBA ESTERS (UNII: CH428W5O62) TRICAPRIN (UNII: O1PB8EU98M) PANTHENOL (UNII: WV9CM0O67Z) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ALOE VERA LEAF (UNII: ZY81Z83H0X) ZOSTERA MARINA WHOLE (UNII: 5JFW9Q62HN) MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) OAT (UNII: Z6J799EAJK) ACAI (UNII: 46AM2VJ0AW) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) SODIUM LACTATE (UNII: TU7HW0W0QT) LEVOMENOL (UNII: 24WE03BX2T) HYDROLYZED GLYCOSAMINOGLYCANS (BOVINE; 50000 MW) (UNII: 997385V0VV) UREA (UNII: 8W8T17847W) BOVINE TYPE I COLLAGEN (UNII: FHJ3ATL51C) LACTIC ACID (UNII: 33X04XA5AT) PHENOXYETHANOL (UNII: HIE492ZZ3T) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ACETYL HEXAPEPTIDE-49 (UNII: 4055X1S509) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54622-301-01 2 in 1 BOX 10/13/2016 1 950 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 09/09/2016 Labeler - Georgia-Pacific Consumer Products (806142217) Registrant - CYAN Labs S.A. de C.V. (812754130) Establishment Name Address ID/FEI Business Operations CYAN Labs S.A. de C.V. 812754130 manufacture(54622-301) , label(54622-301) , pack(54622-301)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.