Unnaplast® with Calamine
Unnaplast with Calamine by
Drug Labeling and Warnings
Unnaplast with Calamine by is a Otc medication manufactured, distributed, or labeled by Pharmaplast SAE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
UNNAPLAST WITH CALAMINE- zinc oxide, calamine dressing
Pharmaplast SAE
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
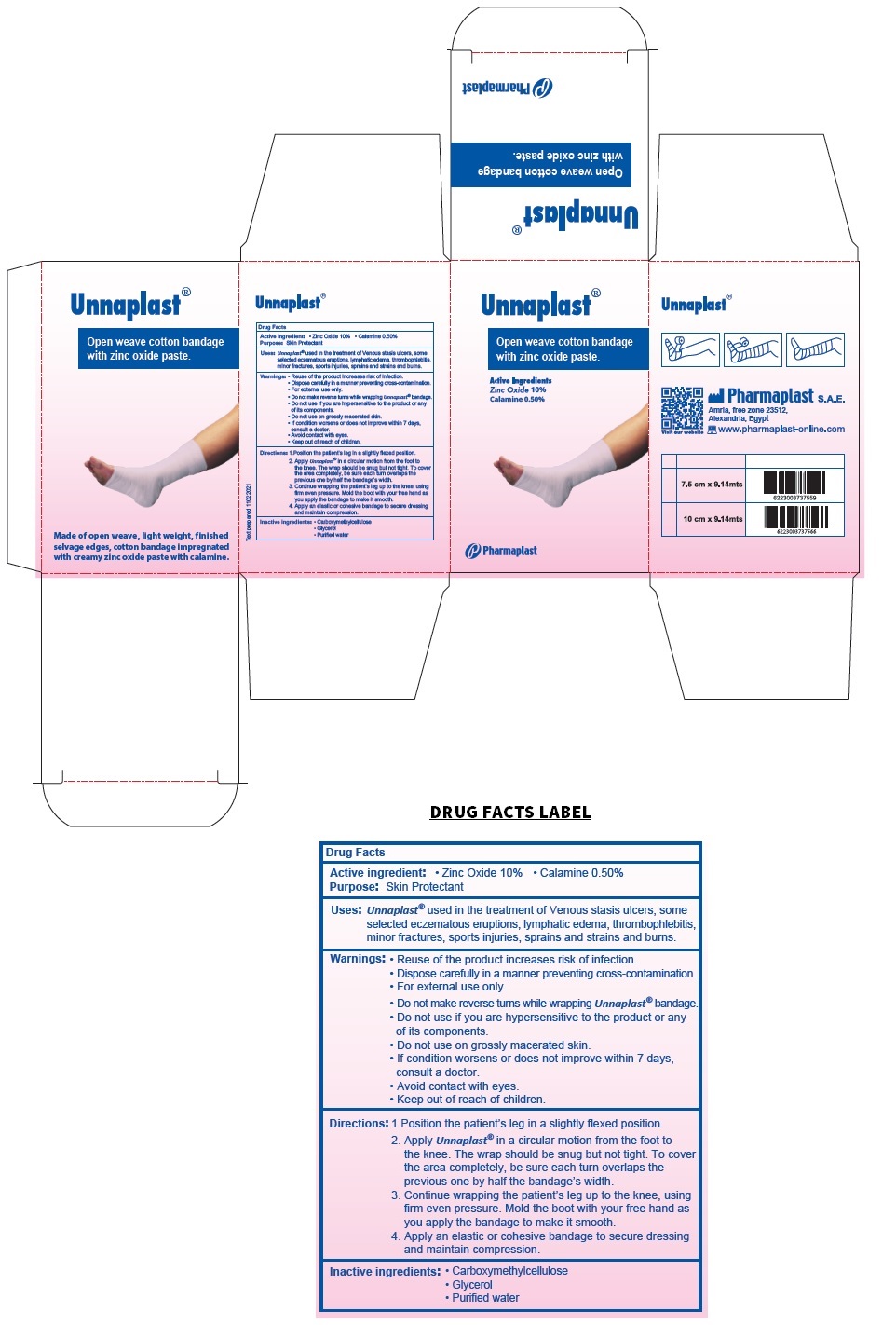
Unnaplast® with Calamine
Uses:
Unnaplast® used in the treatment of Venous stasis ulcers, some selected eczematous eruptions, lymphatic edema, thrombophlebitis, minor fractures, sports injuries, sprains and strains and burns.
Warnings:
Reuse of the product increases risk of infection.
Dispose carefully in a manner preventing cross-contamination.
For external use only.
Do not make reverse turns while wrapping Unnaplast® bandage.
Do not use if you are hypersensitive to the product or any of its components.
Do not use on grossly macerated skin.
If condition worsens or does not improve within 7 days, consult a doctor.
Avoid contact with eyes.
Directions:
1. Position the patient’s leg in a slightly flexed position.
2. Apply Unnaplast® in a circular motion from the foot to the knee. The wrap should be snug but not tight. To cover the area completely, be sure each turn overlaps the previous one by half the bandage’s width.
3. Continue wrapping the patient’s leg up to the knee, using firm even pressure. Mold the boot with your free hand as you apply the bandage to make it smooth.
4. Apply an elastic or cohesive bandage to secure dressing and maintain compression.
| UNNAPLAST WITH CALAMINE
zinc oxide, calamine dressing |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Pharmaplast SAE (644773319) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmaplast SAE | 644773319 | manufacture(28691-1005) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.