CERAVE BABY MOISTURIZING- dimethicone lotion
CeraVe by
Drug Labeling and Warnings
CeraVe by is a Otc medication manufactured, distributed, or labeled by Valeant Pharmaceuticals North America LLC, Product Quest Mfg, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
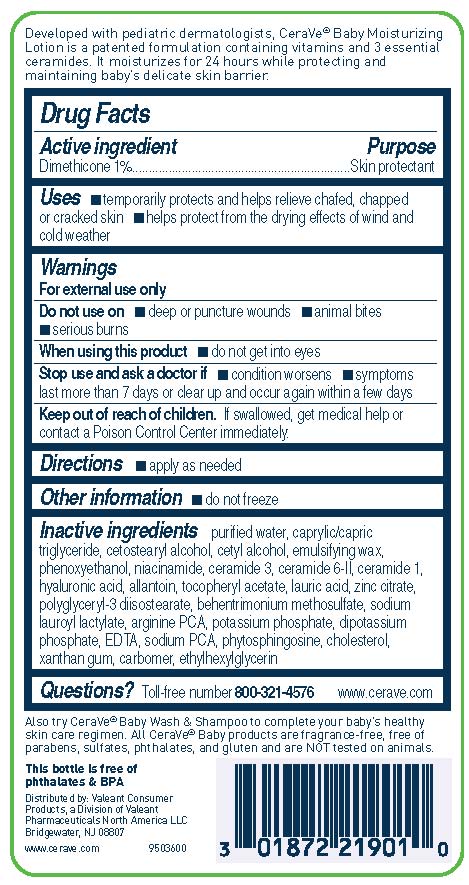
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children.
- Directions
-
Inactive ingredients
purified water, caprylic/capric triglyceride, cetostearyl alcohol, cetyl alcohol, emulsifying wax, phenoxyethanol,niacinamide, ceramide 3, ceramide 6-II, ceramide 1, hyaluronic acid, allantoin, tocopheryl acetate, lauric acid, zinc citrate, polyglyceryl-3 diisostearate, bBehentrimonium methosulfate, sodium lauroyl lactylate, arginine PCA, potassium phosphate, dipotassium phosphate, EDTA, sodium PCA, phytosphingosine, cholesterol, xanthan gum, carbomer, ethylhexylglycerin
- Package/Label Principal Display Panel
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CERAVE BABY MOISTURIZING
dimethicone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0187-2219 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL ALCOHOL (UNII: 936JST6JCN) PHENOXYETHANOL (UNII: HIE492ZZ3T) NIACINAMIDE (UNII: 25X51I8RD4) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) HYALURONIC ACID (UNII: S270N0TRQY) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LAURIC ACID (UNII: 1160N9NU9U) ZINC CITRATE (UNII: K72I3DEX9B) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) EDETIC ACID (UNII: 9G34HU7RV0) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CHOLESTEROL (UNII: 97C5T2UQ7J) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0187-2219-01 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2013 2 NDC: 0187-2219-02 29 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 12/01/2013 Labeler - Valeant Pharmaceuticals North America LLC (042230623) Establishment Name Address ID/FEI Business Operations Product Quest Mfg, LLC 927768135 MANUFACTURE(0187-2219)
Trademark Results [CeraVe]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CERAVE 98843091 not registered Live/Pending |
Yan Run 2024-11-08 |
 CERAVE 98765114 not registered Live/Pending |
Du, Ting 2024-09-23 |
 CERAVE 98733953 not registered Live/Pending |
Haijiang Wang 2024-09-05 |
 CERAVE 98428743 not registered Live/Pending |
Zheng, XiaoLi 2024-03-01 |
 CERAVE 97255760 not registered Live/Pending |
L'Oreal USA Creative, Inc. 2022-02-07 |
 CERAVE 78519354 3234519 Live/Registered |
L'Oreal USA Creative, Inc. 2004-11-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.