Mary Kay Suncare Lip Protector Sunscreen Broad Spectrum SPF 15 Drug Facts
Mary Kay Suncare Lip Protector Sunscreen Broad Spectrum SPF 15 by
Drug Labeling and Warnings
Mary Kay Suncare Lip Protector Sunscreen Broad Spectrum SPF 15 by is a Otc medication manufactured, distributed, or labeled by Mary Kay Inc., Beauty Manufacturing Solutions Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
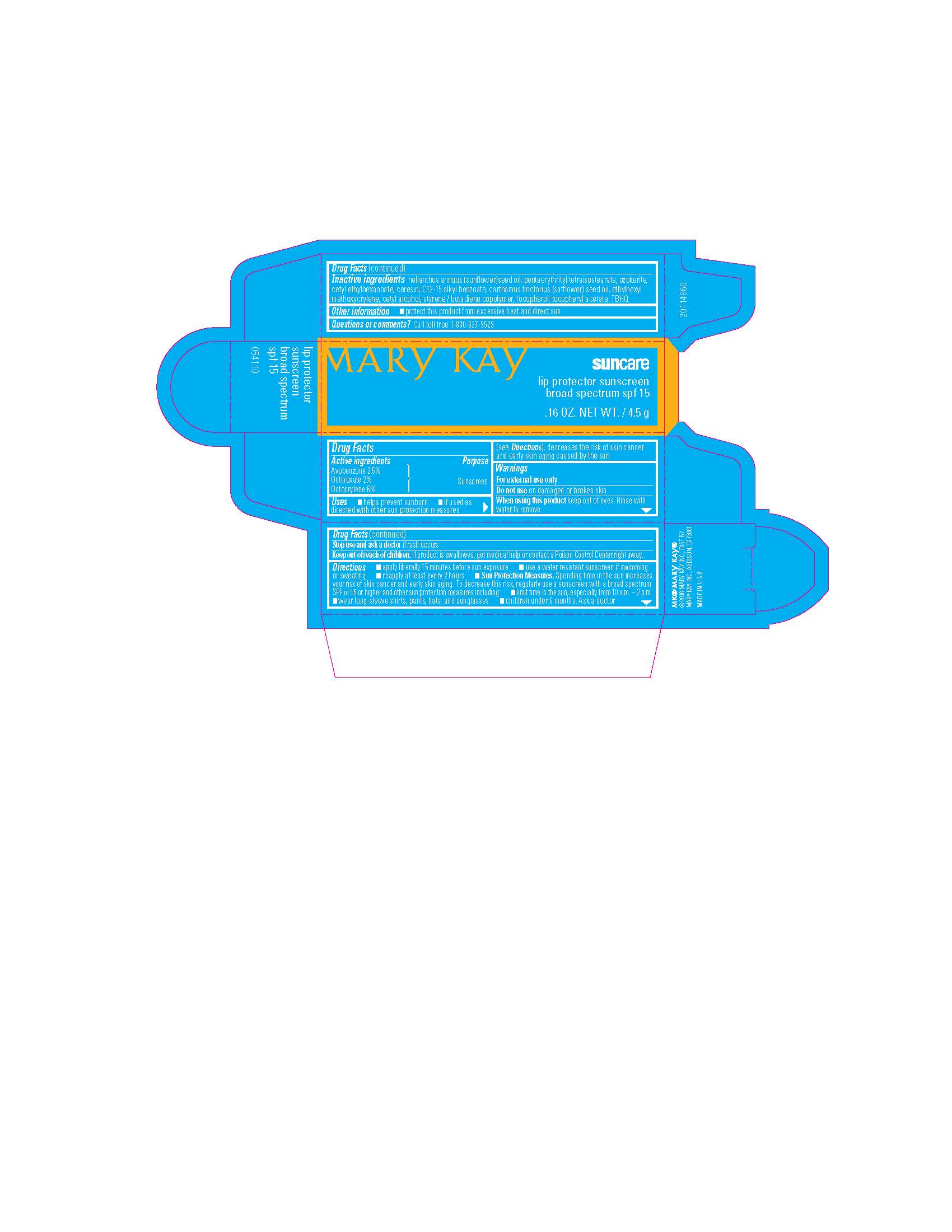
MARY KAY SUNCARE LIP PROTECTOR SUNSCREEN BROAD SPECTRUM SPF 15- sunscreen lipstick
Mary Kay Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Mary Kay Suncare Lip Protector Sunscreen Broad Spectrum SPF 15
Drug Facts
Uses
- helps prevent sunburn
- if used as directed with other su,n protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every two hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF 0f 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- children under 6 months: Ask a doctor.
Inactive ingredients
helianthus annuus (sunflower) seed oil, pentaerythrityl tetraisostearate, ozokerite, cetyl ethylhexanoate, ceresin, C12-15 alkyl benzoate, carthamus tinctorius (safflower) seed oil, ethylhexyl methoxycrylene, cetyl alcohol, styrene/butadiene copolymer, tocopherol, tocopheryl acetate, TBHQ
| MARY KAY SUNCARE LIP PROTECTOR SUNSCREEN BROAD SPECTRUM SPF 15
sunscreen lipstick |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Mary Kay Inc. (049994452) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Beauty Manufacturing Solutions Corp. | 783200723 | manufacture(51531-4110) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.