OXYCONTIN- oxycodone hydrochloride tablet, film coated, extended release
OxyContin by
Drug Labeling and Warnings
OxyContin by is a Prescription medication manufactured, distributed, or labeled by Rebel Distributors Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OXYCONTIN® safely and effectively. See full prescribing information for OXYCONTIN.
OxyContin® (oxycodone hydrochloride controlled-release) Tablets CII
Initial U.S. Approval: 1950WARNING: IMPORTANCE OF PROPER PATIENT SELECTION AND POTENTIAL FOR ABUSE
See full prescribing information for complete boxed warning.- OxyContin contains oxycodone which is an opioid agonist and a Schedule II controlled substance with an abuse liability similar to morphine. (9)
- OxyContin is indicated for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. (1)
- OxyContin is NOT intended for use on an as-needed basis. (1)
- OxyContin 60 mg and 80 mg Tablets, a single dose greater than 40 mg, or a total daily dose greater than 80 mg are only for use in opioid-tolerant patients to avoid fatal respiratory depression. (2.7)
- Patients should be assessed for their clinical risks for opioid abuse or addiction prior to being prescribed opioids. (2.2)
- OxyContin tablets must be swallowed whole and must not be cut, broken, chewed, crushed, or dissolved which can lead to rapid release and absorption of a potentially fatal dose of oxycodone. (2.1)
- The concomitant use with cytochrome P450 3A4 inhibitors such as macrolide antibiotics and protease inhibitors may result in an increase in oxycodone plasma concentrations and may cause potentially fatal respiratory depression. (7.2)
RECENT MAJOR CHANGES
Dosage and Administration
Safe Administration Instructions (2.1)11/2010 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Use low initial doses in patients who are not already opioid-tolerant, especially those who are receiving concurrent treatment with muscle relaxants, sedatives, or other central nervous system (CNS) active medications. (2.2)
- For patients already receiving opioids, use standard conversion ratio estimates. (2.2)
- Tablets must be swallowed whole and are not to be cut, broken, chewed, crushed, or dissolved (risk of potentially fatal dose). (2.1)
- OxyContin tablets should be taken one tablet at a time, with enough water to ensure complete swallowing immediately after placing in the mouth. (2.1, 17.1)
DOSAGE FORMS AND STRENGTHS
- Controlled-Release Tablets: 10 mg, 15 mg, 20 mg , 30 mg, 40 mg, 60 mg and 80 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Must be swallowed whole (5.1)
- May cause somnolence, dizziness, alterations in judgment and alterations in levels of consciousness, including coma. (5.2)
- Additive CNS effects are expected when used with alcohol, other opioids, or illicit drugs. (5.1, 5.3, 7.3)
- Use with caution in patients who are receiving other CNS depressants. (5.1, 5.3, 7.3)
- May cause respiratory depression, use with extreme caution in patients at risk of respiratory depression, elderly and debilitated patients (5.4)
- May aggravate convulsions in patients with convulsive disorders, and may induce or aggravate seizures in some clinical settings. (5.5)
- May worsen increased intracranial pressure and obscure its signs, such as level of consciousness or pupillary signs. (5.6)
- May cause hypotension, use with caution in patients at increased risk of hypotension and in patients in circulatory shock. (5.7)
- Concomitant use of CYP3A4 inhibitors may increase opioid effects (5.8)
- Mixed agonist/antagonist analgesics may precipitate withdrawal symptoms. (5.9)
- Use with caution in patients with biliary tract disease, including acute pancreatitis. (5.10)
- Use with caution in patients at risk for ileus. Monitor for decreased bowel motility in postoperative patients. (5.10)
- Tolerance may develop. (5.11)
- Use with caution in alcoholism; adrenocortical insufficiency; hypothyroidism; prostatic hypertrophy or urethral stricture; severe impairment of hepatic, pulmonary or renal function; and toxic psychosis. (5.12)
- May impair the mental and physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. (5.13)
- No approved use in the treatment of addiction. (5.14)
- Not every urine drug test for "opioids" or "opiates" detects oxycodone reliably. (5.15)
ADVERSE REACTIONS
Most common adverse reactions (>5%) are constipation, nausea, somnolence, dizziness, vomiting, pruritus, headache, dry mouth, asthenia, and sweating.
To report Suspected Adverse Reactions, contact Purdue Pharma L.P. at 1-888-726-7535 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)DRUG INTERACTIONS
- OxyContin may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. (7.1)
- The CYP3A4 isoenzyme plays a major role in the metabolism of OxyContin, drugs that inhibit CYP3A4 activity may cause decreased clearance of oxycodone which could lead to an increase in oxycodone plasma concentrations. (7.2)
- Concurrent use of other CNS depressants may cause respiratory depression, hypotension, and profound sedation or coma. (7.3)
- Mixed agonist/antagonist analgesics may reduce the analgesic effect of oxycodone and may precipitate withdrawal symptoms in these patients. (7.4)
USE IN SPECIFIC POPULATIONS
- Labor and Delivery: Not recommended for use in women immediately prior to and during labor and delivery; (8.2)
- Nursing Mothers: Nursing should not be undertaken while a patient is receiving OxyContin. (8.3)
- Pediatrics: Safety and effectiveness in pediatric patients below the age of 18 have not been established. (8.4)
- Geriatrics: The initial dose may need to be reduced to 1/3 to ½ of the usual doses. (8.5)
- Hepatic impairment: Initiate therapy at 1/3 to 1/2 the usual doses and titrate carefully. (8.6)
- Renal impairment: Dose initiation should follow a conservative approach. (8.7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: IMPORTANCE OF PROPER PATIENT SELECTION AND POTENTIAL FOR ABUSE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Safe Administration Instructions
2.2 Initiating Therapy with OxyContin
2.3 Conversion from Transdermal Fentanyl to OxyContin
2.4 Hepatic Impairment
2.5 Managing Expected Opioid Adverse Reactions
2.6 Individualization of Dosage
2.7 Special Instructions for Patients who are not Opioid Tolerant
2.8 Continuation of Therapy
2.9 Cessation of Therapy
2.10 Conversion from OxyContin to Parenteral Opioids
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Information Essential for Safe Administration
5.2 CNS Depression
5.3 Interactions with Alcohol, CNS Depressants and Illicit Drugs
5.4 Respiratory Depression
5.5 Seizures
5.6 Head Injury
5.7 Hypotensive Effect
5.8 Cytochrome P450 3A4 Inhibitors and Inducers
5.9 Interactions with Mixed Agonist/Antagonist Opioid Analgesics
5.10 Use in Pancreatic/Biliary Tract Disease and Other Gastrointestinal Conditions
5.11 Tolerance
5.12 Special Risk Groups
5.13 Driving and Operating Machinery
5.14 Use in Addiction Treatment
5.15 Laboratory Monitoring
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Neuromuscular Junction Blocking Agents
7.2 Agents Affecting Cytochrome P450 Isoenzymes
7.3 CNS Depressants
7.4 Interactions with Mixed Agonist/Antagonist Opioid Analgesics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 Gender Differences
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients and Caregivers
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: IMPORTANCE OF PROPER PATIENT SELECTION AND POTENTIAL FOR ABUSE
OxyContin contains oxycodone which is an opioid agonist and a Schedule II controlled substance with an abuse liability similar to morphine. (9)
OxyContin can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing OxyContin in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion. (9.2)
OxyContin is a controlled-release oral formulation of oxycodone hydrochloride indicated for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. (1)
OxyContin is not intended for use on an as-needed basis. (1)
Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine/day, 25 mcg transdermal fentanyl/hour, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day, or an equianalgesic dose of another opioid for one week or longer.
OxyContin 60 mg and 80 mg tablets, a single dose greater than 40 mg, or a total daily dose greater than 80 mg are only for use in opioid-tolerant patients, as they may cause fatal respiratory depression when administered to patients who are not tolerant to the respiratory-depressant or sedating effects of opioids. (2.7)Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). Patients should be assessed for their clinical risks for opioid abuse or addiction prior to being prescribed opioids. All patients receiving opioids should be routinely monitored for signs of misuse, abuse and addiction. (2.2)
OxyContin must be swallowed whole and must not be cut, broken, chewed, crushed, or dissolved. Taking cut, broken, chewed, crushed or dissolved OxyContin tablets leads to rapid release and absorption of a potentially fatal dose of oxycodone. (2.1)
The concomitant use of OxyContin with all cytochrome P450 3A4 inhibitors such as macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), and protease inhibitors (e.g., ritonavir) may result in an increase in oxycodone plasma concentrations, which could increase or prolong adverse effects and may cause potentially fatal respiratory depression. Patients receiving OxyContin and a CYP3A4 inhibitor should be carefully monitored for an extended period of time and dosage adjustments should be made if warranted. (7.2)
-
1 INDICATIONS AND USAGE
OxyContin is a controlled-release oral formulation of oxycodone hydrochloride indicated for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time.
Limitations of Usage
OxyContin is not intended for use on an as-needed basis.
OxyContin is not indicated for the management of pain in the immediate postoperative period (the first 12-24 hours following surgery), or if the pain is mild, or not expected to persist for an extended period of time. OxyContin is indicated for postoperative use following the immediate post-operative period only if the patient is already receiving the drug prior to surgery or if the postoperative pain is expected to be moderate to severe and persist for an extended period of time. Physicians should individualize treatment, moving from parenteral to oral analgesics as appropriate. (See American Pain Society guidelines.)
OxyContin is not indicated for pre-emptive analgesia (preoperative administration for the management of postoperative pain).
OxyContin is not indicated for rectal administration.
-
2 DOSAGE AND ADMINISTRATION
2.1 Safe Administration Instructions
OxyContin tablets must be swallowed whole and must not be cut, broken, chewed, crushed or dissolved. Taking cut, broken, chewed, crushed or dissolved OxyContin tablets leads to rapid release and absorption of a potentially fatal dose of oxycodone.
OxyContin tablets should be taken one tablet at a time. Take each tablet with enough water to ensure complete swallowing immediately after placing in the mouth [see Patient Counseling Information (17.1)].
Selection of patients for treatment with OxyContin should be governed by the same principles that apply to the use of similar opioid analgesics. Physicians should individualize treatment using a progressive plan of pain management such as outlined by the World Health Organization, Federation of State Medical Boards Model Policy, and the American Pain Society. Healthcare professionals should follow appropriate pain management principles of careful assessment and ongoing monitoring.
2.2 Initiating Therapy with OxyContin
It is critical to initiate the dosing regimen for each patient individually. Attention should be given to:
- risk factors for abuse or addiction; including whether the patient has a previous or current substance abuse problem, a family history of substance abuse, or a history of mental illness or depression;
- the age, general condition and medical status of the patient;
- the patient's opioid exposure and opioid tolerance (if any);
- the daily dose, potency, and kind of the analgesic(s) the patient has been taking;
- the reliability of the conversion estimate used to calculate the dose of oxycodone;
- the special instructions for OxyContin 60 mg and 80 mg tablets, a single dose greater than 40 mg, or total daily doses greater than 80 mg [see Dosage and Administration (2.7)]; and
- the balance between pain control and adverse reactions.
Use low initial doses of OxyContin in patients who are not already opioid-tolerant [see Dosage and Administration (2.7)], especially those who are receiving concurrent treatment with muscle relaxants, sedatives, or other CNS active medications [see Warnings and Precautions (5.1, 5.3) and Drug Interactions (7.1, 7.3)].
Experience indicates a reasonable starting dose of OxyContin for patients who are taking non-opioid analgesics and require continuous around-the-clock therapy for an extended period of time is 10 mg every 12 hours. Individually titrate OxyContin to a dose that provides adequate analgesia and minimizes adverse reactions while maintaining an every-twelve-hour dosing regimen.
For initiation of OxyContin therapy for patients previously taking opioids, the conversion ratios found in Table 1 are a reasonable starting point, although not verified in well-controlled, multiple-dose trials. No fixed conversion ratio is likely to be satisfactory in all patients, especially patients receiving large opioid doses. A reasonable approach for converting from existing opioid therapy to OxyContin is as follows:
- Discontinue all other around-the-clock opioid drugs when OxyContin therapy is initiated.
- Using standard conversion ratio estimates (see Table 1), multiply the mg/day of each of the current opioids to be converted by their appropriate multiplication factor to obtain the equivalent total daily dose of oral oxycodone.
- Divide the calculated 24-hour oxycodone dose in half to approximate the every 12-hour dose of OxyContin.
- Round down, if necessary, to the appropriate OxyContin tablet strengths available.
- Close observation and frequent titration are indicated until patients are stable on the new therapy.
TABLE 1 Multiplication Factors for Converting the Daily Dose
of Current Opioids to the Daily Dose of Oral Oxycodone1** To be used only for conversion to oral oxycodone. For patients receiving high-dose parenteral opioids, a more conservative conversion is warranted. For example, for high-dose parenteral morphine, use 1.5 instead of 3 as a multiplication factor. (mg/Day Opioid x Factor = mg/Day Oral Oxycodone) Oral Opioid Parenteral Opioid Oxycodone 1 -- Codeine 0.15 -- Hydrocodone 0.9 -- Hydromorphone 4 20 Levorphanol 7.5 15 Meperidine 0.1 0.4 Methadone 1.5 3 Morphine 0.5 3 2.3 Conversion from Transdermal Fentanyl to OxyContin
Eighteen hours following the removal of the transdermal fentanyl patch, OxyContin treatment can be initiated. Although there has been no systematic assessment of such conversion, a conservative oxycodone dose, approximately 10 mg every 12 hours of OxyContin, should be initially substituted for each 25 mcg/hr fentanyl transdermal patch. Follow the patient closely during conversion from transdermal fentanyl to OxyContin, as there is limited documented experience with this conversion.
2.4 Hepatic Impairment
For patients with hepatic impairment, start dosing patients at 1/3 to 1/2 the usual starting dose followed by careful dose titration [see Clinical Pharmacology (12.3)].
2.5 Managing Expected Opioid Adverse Reactions
Most patients receiving OxyContin, especially those who are opioid-naive, will experience adverse reactions. Patients do not usually become tolerant to the constipating effects of opioids, therefore, anticipate constipation and treat aggressively and prophylactically with a stimulant laxative with or without a stool softener.
If nausea persists and is unacceptable to the patient, consider treatment with antiemetics or other modalities to relieve these symptoms.
2.6 Individualization of Dosage
Once therapy is initiated, assess pain relief and other opioid effects frequently. Titrate patients to adequate effect (generally mild or no pain with the regular use of no more than two doses of supplemental analgesia per 24 hours). Patients who experience breakthrough pain may require dosage adjustment or rescue medication. Because steady-state plasma concentrations are approximated within 24 to 36 hours, dosage adjustment may be carried out every 1 to 2 days.
There are no well-controlled clinical studies evaluating the safety and efficacy with dosing more frequently than every 12 hours. Increase the OxyContin dose by increasing the total daily dose, not by changing the 12-hour dosing interval. As a guideline, the total daily oxycodone dose usually can be increased by 25% to 50% of the current dose, each time an increase is clinically indicated.
If signs of excessive opioid-related adverse reactions are observed, the next dose may be reduced. If this adjustment leads to inadequate analgesia, a supplemental dose of immediate-release oxycodone may be given. Alternatively, non-opioid analgesic adjuvants may be employed. Adjust the dose to obtain an appropriate balance between pain relief and opioid-related adverse reactions.
During periods of changing analgesic requirements, including initial titration, maintain frequent contact between physician, other members of the healthcare team, the patient and, with proper consent, the caregiver/family.
2.7 Special Instructions for Patients who are not Opioid Tolerant
Do not begin treatment with OxyContin 60 mg and 80 mg Tablets, a single dose greater than 40 mg, or a total daily dose greater than 80 mg in patients who are not already tolerant to the respiratory-depressant and sedating effects of opioids. Use of these doses in patients who are not opioid tolerant may cause fatal respiratory depression. These doses are only for use in opioid-tolerant patients.
Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine/day, 25 mcg transdermal fentanyl/hour, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day, or an equianalgesic dose of another opioid for one week or longer.
Instruct patients not to share or permit use by individuals other than the patient for whom OxyContin was prescribed, as such inappropriate use may have severe medical consequences, including death.
2.8 Continuation of Therapy
During chronic therapy, especially for non-cancer pain syndromes, reassess the continued need for around-the-clock opioid therapy regularly (e.g., every 6 to 12 months) as appropriate.
-
3 DOSAGE FORMS AND STRENGTHS
- 10 mg film-coated tablets (round, white-colored, bi-convex tablets debossed with OP on one side and 10 on the other)
- 15 mg film-coated tablets (round, gray-colored, bi-convex tablets debossed with OP on one side and 15 on the other)
- 20 mg film-coated tablets (round, pink-colored, bi-convex tablets debossed with OP on one side and 20 on the other)
- 30 mg film-coated tablets (round, brown-colored, bi-convex tablets debossed with OP on one side and 30 on the other)
- 40 mg film-coated tablets (round, yellow-colored, bi-convex tablets debossed with OP on one side and 40 on the other)
- 60 mg film-coated tablets* (round, red-colored, bi-convex tablets debossed with OP on one side and 60 on the other)
- 80 mg film-coated tablets* (round, green-colored, bi-convex tablets debossed with OP on one side and 80 on the other)
* 60 mg and 80 mg tablets for use in opioid-tolerant patients only
-
4 CONTRAINDICATIONS
OxyContin is contraindicated in:
- patients who have significant respiratory depression
- patients who have or are suspected of having paralytic ileus
- patients who have acute or severe bronchial asthma
- patients who have known hypersensitivity to any of its components or the active ingredient, oxycodone.
-
5 WARNINGS AND PRECAUTIONS
5.1 Information Essential for Safe Administration
OxyContin tablets must be swallowed whole and must not be cut, broken, chewed, crushed, or dissolved. Taking cut, broken, chewed, crushed or dissolved OxyContin tablets leads to rapid release and absorption of a potentially fatal dose of oxycodone.
OxyContin 60 mg and 80 mg Tablets, a single dose greater than 40 mg, or a total daily dose greater than 80 mg are only for use in opioid-tolerant patients. Use of these doses in patients who are not opioid tolerant may cause fatal respiratory depression.
Instruct patients against use by individuals other than the patient for whom OxyContin was prescribed, as such inappropriate use may have severe medical consequences, including death.
Opioid analgesics have a narrow therapeutic index in certain patient populations, especially when combined with CNS depressant drugs, and should be reserved for cases where the benefits of opioid analgesia outweigh the known risks of respiratory depression, altered mental state, and postural hypotension.
5.2 CNS Depression
OxyContin may cause somnolence, dizziness, alterations in judgment and alterations in levels of consciousness, including coma.
5.3 Interactions with Alcohol, CNS Depressants and Illicit Drugs
Hypotension, profound sedation, coma or respiratory depression may result if OxyContin is added to a regimen that includes other CNS depressants (e.g., sedatives, anxiolytics, hypnotics, neuroleptics, other opioids). Therefore, use caution when deciding to initiate therapy with OxyContin in patients who are taking other CNS depressants. Take into account the types of other medications being taken, the duration of therapy with them, and the patient's response to those medicines, including the degree of tolerance that has developed to CNS depression. Consider the patient's use, if any, of alcohol and/or illicit drugs that cause CNS depression. If the decision to begin OxyContin is made, start with a lower OxyContin dose than usual. [see Drug Interactions (7.3)]
Consider using a lower initial dose of a CNS depressant when given to a patient currently taking OxyContin due to the potential of additive CNS depressant effects.
5.4 Respiratory Depression
Decreased respiratory drive resulting in respiratory depression is the chief hazard from the use or abuse of opioid agonists, including OxyContin. The risk of opioid-induced respiratory depression is increased, for example, in elderly [see Use In Specific Populations (8.5)] or debilitated patients; following large initial doses in any patient who is not tolerant to the respiratory-depressant or sedating effects of opioids; or when opioids are given in conjunction with other agents that either depress respiratory drive or consciousness.
Use OxyContin with extreme caution in patients with any of the following:
- significant chronic obstructive pulmonary disease or cor pulmonale
- other risk of substantially decreased respiratory reserve
- hypoxia
- hypercapnia
- pre-existing respiratory depression
Respiratory depression induced by opioids typically follows a pattern entailing first a shift in CO2 responsiveness of the CNS respiratory drive center, which results in a decrease in the urge to breathe, despite the presence of hypercapnia. The increase in brain CO2 can result in sedation that can accentuate the sedation from the opioid itself. Profound sedation, unresponsiveness, infrequent deep ("sighing") breaths or atypical snoring frequently accompany opioid-induced respiratory depression. Eventually, hypoxia ensues. In addition to further decreasing consciousness, hypoxia, along with hypercapnia, can predispose to life-threatening cardiac arrhythmias.
5.5 Seizures
Oxycodone, as with other opioids, may aggravate convulsions in patients with convulsive disorders, and may induce or aggravate seizures in some clinical settings. Use OxyContin with caution in patients with a history of seizure disorders.
5.6 Head Injury
The respiratory depressant effects of opioids include carbon dioxide retention, which can lead to an elevation of cerebrospinal fluid pressure. This effect may be exaggerated in the presence of head injury, intracranial lesions, or other sources of pre-existing increased intracranial pressure. Oxycodone may produce miosis that is independent of ambient light, and altered consciousness, either of which may obscure neurologic signs associated with increased intracranial pressure in persons with head injuries.
5.7 Hypotensive Effect
OxyContin may cause severe hypotension. There is an added risk to individuals whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines or other agents which compromise vasomotor tone. Oxycodone may produce orthostatic hypotension in ambulatory patients. Administer OxyContin with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
5.8 Cytochrome P450 3A4 Inhibitors and Inducers
Since the CYP3A4 isoenzyme plays a major role in the metabolism of OxyContin, drugs that alter CYP3A4 activity may cause changes in clearance of oxycodone which could lead to changes in oxycodone plasma concentrations.
The expected clinical results with CYP3A4 inhibitors would be an increase in oxycodone plasma concentrations and possibly increased or prolonged opioid effects. The expected clinical results with CYP3A4 inducers would be a decrease in oxycodone plasma concentrations, lack of efficacy or, possibly, development of an abstinence syndrome in a patient who had developed physical dependence to oxycodone.
If co-administration is necessary, caution is advised when initiating OxyContin treatment in patients currently taking, or discontinuing, CYP3A4 inhibitors or inducers. Evaluate these patients at frequent intervals and consider dose adjustments until stable drug effects are achieved [see Drug Interactions (7.2) and Clinical Pharmacology (12)].
5.9 Interactions with Mixed Agonist/Antagonist Opioid Analgesics
It is generally not advisable to administer mixed agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, and butorphanol) to a patient receiving OxyContin. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect and may precipitate withdrawal symptoms in these patients.
5.10 Use in Pancreatic/Biliary Tract Disease and Other Gastrointestinal Conditions
Oxycodone may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis. Opioids may cause increases in the serum amylase.
The administration of OxyContin may obscure the diagnosis or clinical course in patients with acute abdominal conditions. Use OxyContin with caution in patients who are at risk of developing ileus.
5.11 Tolerance
Tolerance to opioids is demonstrated by the need for increasing doses to maintain adequate analgesic effect (in the absence of disease progression or other external factors). If tolerance develops, or if pain severity increases, a gradual increase in dose may be required. The first sign of tolerance is usually a reduced duration of effect. Tolerance to different effects of opioids may develop to varying degrees and at varying rates in a given individual. There is also inter-patient variability in the rate and extent of tolerance that develops to various opioid effects, whether the effect is desirable (e.g., analgesia) or undesirable (e.g., nausea).
5.12 Special Risk Groups
Use OxyContin with caution in the following conditions, due to increased risk of adverse reactions: alcoholism; delirium tremens; adrenocortical insufficiency; CNS depression; debilitation; kyphoscoliosis associated with respiratory compromise; myxedema or hypothyroidism; prostatic hypertrophy or urethral stricture; severe impairment of hepatic, pulmonary or renal function; and toxic psychosis.
5.13 Driving and Operating Machinery
OxyContin may impair the mental and physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Caution patients accordingly.
5.14 Use in Addiction Treatment
OxyContin has no approved use in the treatment of addiction. Its proper usage in individuals with drug or alcohol addiction (substance dependence), either active or in remission, is for the management of pain requiring opioid analgesia.
5.15 Laboratory Monitoring
Not every urine drug test for "opioids" or "opiates" detects oxycodone reliably, especially those designed for in-office use. Further, many laboratories will report urine drug concentrations below a specified "cut-off" value as "negative". Therefore, if urine testing for oxycodone is considered in the clinical management of an individual patient, ensure that the sensitivity and specificity of the assay is appropriate, and use caution in interpreting results.
-
6 ADVERSE REACTIONS
The following adverse reactions described elsewhere in the labeling include:
- Respiratory depression [see Boxed Warning, Warnings and Precautions (5.1, 5.4) and Overdosage (10)]
- CNS depression [see Warnings and Precautions (5.1, 5.2) and Overdosage (10)]
- Hypotensive effects [see Warning and Precautions (5.7) and Overdosage (10)]
- Drug abuse, addiction, and dependence [see Drug Abuse and Dependence (9.2, 9.3)]
- Paralytic ileus [see Warnings and Precautions (5.10)]
- Seizures [see Warnings and Precautions (5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of OxyContin was evaluated in double-blind clinical trials involving 713 patients with moderate to severe pain of various etiologies. In open-label studies of cancer pain, 187 patients received OxyContin in total daily doses ranging from 20 mg to 640 mg per day. The average total daily dose was approximately 105 mg per day.
OxyContin may increase the risk of serious adverse reactions such as those observed with other opioid analgesics, including respiratory depression, apnea, respiratory arrest, circulatory depression, hypotension, or shock [see Overdosage (10)].
The most common adverse reactions (>5%) reported by patients in clinical trials comparing OxyContin with placebo are shown in Table 2 below:
TABLE 2: Common Adverse Reactions (>5%) Adverse
ReactionOxyContin
(n=227)Placebo
(n=45)(%) (%) Constipation (23) (7) Nausea (23) (11) Somnolence (23) (4) Dizziness (13) (9) Pruritus (13) (2) Vomiting (12) (7) Headache (7) (7) Dry Mouth (6) (2) Asthenia (6) - Sweating (5) (2) In clinical trials, the following adverse reactions were reported in patients treated with OxyContin with an incidence between 1% and 5%:
Gastrointestinal disorders: abdominal pain, diarrhea, dyspepsia, gastritis, hiccups
General disorders and administration site conditions: chills, fever
Metabolism and nutrition disorders: anorexia
Musculoskeletal and connective tissue disorders: twitching
Psychiatric disorders: abnormal dreams, anxiety, confusion, dysphoria, euphoria, insomnia, nervousness, thought abnormalities
Respiratory, thoracic and mediastinal disorders: dyspnea, hiccups
Skin and subcutaneous tissue disorders: rash
Vascular disorders: postural hypotension
The following adverse reactions occurred in less than 1% of patients involved in clinical trials:
Blood and lymphatic system disorders: lymphadenopathy
Ear and labyrinth disorders: tinnitus
Eye disorders: abnormal vision
Gastrointestinal disorders: dysphagia, eructation, flatulence, gastrointestinal disorder, increased appetite, stomatitis
General disorders and administration site conditions: withdrawal syndrome (with and without seizures), edema, peripheral edema, thirst, malaise, chest pain, facial edema
Injury, poisoning and procedural complications: accidental injury
Investigations: ST depression
Metabolism and nutrition disorders: dehydration
Nervous system disorders: syncope, migraine, abnormal gait, amnesia, hyperkinesia, hypesthesia, hypotonia, paresthesia, speech disorder, stupor, tremor, vertigo, taste perversion
Psychiatric disorders: depression, agitation, depersonalization, emotional lability, hallucination
Renal and urinary disorders: dysuria, hematuria, polyuria, urinary retention
Reproductive system and breast disorders: impotence
Respiratory, thoracic and mediastinal disorders: cough increased, voice alteration
Skin and subcutaneous tissue disorders: dry skin, exfoliative dermatitis
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of controlled-release oxycodone. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: abuse, addiction, overdose, death, amenorrhea, symptoms associated with an anaphylactic or anaphylactoid reaction, cholestasis, dental caries, increased hepatic enzymes, muscular hypertonia, hyponatremia, ileus, palpitations (in the context of withdrawal), seizures, syndrome of inappropriate antidiuretic hormone secretion, and urticaria
In addition to the events listed above, the following have also been reported, potentially due to the swelling and hydrogelling property of the tablet: choking, gagging, regurgitation, tablets stuck in the throat and difficulty swallowing the tablet.
-
7 DRUG INTERACTIONS
7.1 Neuromuscular Junction Blocking Agents
OxyContin may enhance the neuromuscular blocking action of true skeletal muscle relaxants (such as pancuronium) and produce an increased degree and/or duration of respiratory depression.
7.2 Agents Affecting Cytochrome P450 Isoenzymes
Inhibitors of CYP3A4:
Since the CYP3A4 isoenzyme plays a major role in the metabolism of OxyContin, drugs that inhibit CYP3A4 activity, such as macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), and protease inhibitors (e.g., ritonavir), may cause decreased clearance of oxycodone which could lead to an increase in oxycodone plasma concentrations. A published study showed that the co-administration of the antifungal drug, voriconazole, increased oxycodone AUC and Cmax by 3.6 and 1.7 fold, respectively. Although clinical studies have not been conducted with other CYP3A4 inhibitors, the expected clinical results would be increased or prolonged opioid effects. If co-administration with OxyContin is necessary, caution is advised when initiating therapy with, currently taking, or discontinuing CYP450 inhibitors. Evaluate these patients at frequent intervals and consider dose adjustments until stable drug effects are achieved. [see Clinical Pharmacology (12.3)]Inducers of CYP3A4:
CYP450 inducers, such as rifampin, carbamazepine, and phenytoin, may induce the metabolism of oxycodone and, therefore, may cause increased clearance of the drug which could lead to a decrease in oxycodone plasma concentrations, lack of efficacy or, possibly, development of an abstinence syndrome in a patient who had developed physical dependence to oxycodone. A published study showed that the co-administration of rifampin, a drug metabolizing enzyme inducer, decreased oxycodone (oral) AUC and Cmax by 86% and 63%, respectively. If co-administration with OxyContin is necessary, caution is advised when initiating therapy with, currently taking, or discontinuing CYP3A4 inducers. Evaluate these patients at frequent intervals and consider dose adjustments until stable drug effects are achieved.Inhibitors of CYP2D6:
Oxycodone is metabolized in part to oxymorphone via cytochrome CYP2D6. While this pathway may be blocked by a variety of drugs (e.g., certain cardiovascular drugs including amiodarone and quinidine as well as polycyclic antidepressants), such blockade has not been shown to be of clinical significance during oxycodone treatment.7.3 CNS Depressants
Start OxyContin at 1/3 to 1/2 of the usual dosage in patients who are concurrently receiving other CNS depressants including sedatives or hypnotics, general anesthetics, phenothiazines, centrally acting anti-emetics, tranquilizers, and alcohol because respiratory depression, hypotension, and profound sedation or coma may result. No specific interaction between oxycodone and monoamine oxidase inhibitors has been observed, but caution in the use of any opioid in patients taking this class of drugs is appropriate. [see Warnings and Precautions (5.2 )]
7.4 Interactions with Mixed Agonist/Antagonist Opioid Analgesics
Mixed agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, and butorphanol) should generally not be administered to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic such as OxyContin. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of oxycodone and may precipitate withdrawal symptoms in these patients.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Category B:
There are no adequate and well-controlled studies of oxycodone use during pregnancy. Based on limited human data in the literature, oxycodone does not appear to increase the risk of congenital malformations. In animal reproduction and developmental toxicology studies, no evidence of fetal harm was observed. Because animal reproduction studies are not always predictive of human response, oxycodone should be used during pregnancy only if clearly needed.
Teratogenic Effects
The effect of oxycodone in human reproduction has not been adequately studied. Studies with oral doses of oxycodone hydrochloride in rats up to 8 mg/kg/day and rabbits up to 125 mg/kg/day, equivalent to 0.5 and 15 times an adult human dose of 160 mg/day, respectively on a mg/m2 basis, did not reveal evidence of harm to the fetus due to oxycodone. In a pre- and postnatal toxicity study, female rats received oxycodone during gestation and lactation. There were no long-term developmental or reproductive effects in the pups. [see Nonclinical Toxicology (13)]
Non-Teratogenic Effects
Oxycodone hydrochloride was administered orally to female rats during gestation and lactation in a pre- and postnatal toxicity study. There were no drug-related effects on reproductive performance in these females or any long-term developmental or reproductive effects in pups born to these rats. Decreased body weight was found during lactation and the early post-weaning phase in pups nursed by mothers given the highest dose used (6 mg/kg/day, equivalent to approximately 0.4-times an adult human dose of 160 mg/day, on a mg/m2 basis). However, body weight of these pups recovered.
8.2 Labor and Delivery
Opioids cross the placenta and may produce respiratory depression and psychophysiologic effects in neonates. OxyContin is not recommended for use in women immediately prior to and during labor, when use of shorter-acting analgesics or other analgesic techniques are more appropriate. Occasionally, opioid analgesics may prolong labor through actions which temporarily reduce the strength, duration and frequency of uterine contractions. However this effect is not consistent and may be offset by an increased rate of cervical dilatation, which tends to shorten labor.
Closely observe neonates whose mothers received opioid analgesics during labor for signs of respiratory depression. Have a specific opioid antagonist, such as naloxone or nalmefene, available for reversal of opioid-induced respiratory depression in the neonate.
Neonates whose mothers have been taking opioids chronically may also exhibit withdrawal signs, either at birth and/or in the nursery, because they have developed physical dependence. This is not, however, synonymous with addiction [see Drug Abuse and Dependence (9.3)]. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening and should be treated according to protocols developed by neonatology experts.
8.3 Nursing Mothers
Oxycodone has been detected in breast milk. Instruct patients not to undertake nursing while receiving OxyContin. Do not initiate OxyContin therapy while nursing because of the possibility of sedation or respiratory depression in the infant.
Withdrawal symptoms can occur in breast-fed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
8.4 Pediatric Use
Safety and effectiveness of OxyContin in pediatric patients below the age of 18 years have not been established.
8.5 Geriatric Use
In controlled pharmacokinetic studies in elderly subjects (greater than 65 years) the clearance of oxycodone was slightly reduced. Compared to young adults, the plasma concentrations of oxycodone were increased approximately 15% [see Clinical Pharmacology (12.3)]. Of the total number of subjects (445) in clinical studies of oxycodone hydrochloride controlled-release tablets, 148 (33.3%) were age 65 and older (including those age 75 and older) while 40 (9.0%) were age 75 and older. In clinical trials with appropriate initiation of therapy and dose titration, no untoward or unexpected adverse reactions were seen in the elderly patients who received oxycodone hydrochloride controlled-release tablets. Thus, the usual doses and dosing intervals may be appropriate for elderly patients. However, reduce the starting dose to 1/3 to ½ the usual dosage in debilitated, non-opioid-tolerant patients. Respiratory depression is the chief risk in elderly or debilitated patients, usually the result of large initial doses in patients who are not tolerant to opioids, or when opioids are given in conjunction with other agents that depress respiration. Titrate the dose of OxyContin cautiously in these patients.
8.6 Hepatic Impairment
A study of OxyContin in patients with hepatic impairment demonstrated greater plasma concentrations than those seen at equivalent doses in persons with normal hepatic function. Therefore, in the setting of hepatic impairment, start dosing patients at 1/3 to 1/2 the usual starting dose followed by careful dose titration [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
In patients with renal impairment, as evidenced by decreased creatinine clearance (<60 mL/min), the concentrations of oxycodone in the plasma are approximately 50% higher than in subjects with normal renal function. Follow a conservative approach to dose initiation and adjust according to the clinical situation [see Clinical Pharmacology (12.3)].
8.8 Gender Differences
In pharmacokinetic studies with OxyContin, opioid-naive females demonstrate up to 25% higher average plasma concentrations and greater frequency of typical opioid adverse events than males, even after adjustment for body weight. The clinical relevance of a difference of this magnitude is low for a drug intended for chronic usage at individualized dosages, and there was no male/female difference detected for efficacy or adverse events in clinical trials.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
OxyContin contains oxycodone, which is a Schedule II controlled substance with an abuse liability similar to morphine. OxyContin, like morphine and other opioids used for analgesia, can be abused and is subject to criminal diversion.
9.2 Abuse
Abuse of OxyContin poses a hazard of overdose and death. This risk is increased with compromising the tablet and with concurrent abuse of alcohol or other substances.
With parenteral abuse, the tablet excipients can result in death, local tissue necrosis, infection, pulmonary granulomas, and increased risk of endocarditis and valvular heart injury. Parenteral drug abuse is commonly associated with transmission of infectious diseases, such as hepatitis and HIV.
Opioid drugs are sought by people with substance use disorders (abuse or addiction, the latter of which is also called "substance dependence") and criminals who supply them by diverting medicines out of legitimate distribution channels. OxyContin is a target for theft and diversion.
"Drug-seeking" behavior is very common in persons with substance use disorders. Drug-seeking tactics include, but are not limited to, emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated "loss" of prescriptions, altering or forging of prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). "Doctor shopping" to obtain additional prescriptions is common among people with untreated substance use disorders, and criminals who divert controlled substances.
The risks of misuse and abuse should be considered when prescribing or dispensing OxyContin. Concerns about abuse and addiction, should not prevent the proper management of pain, however. Treatment of pain should be individualized, balancing the potential benefits and risks for each patient.
Compromising an extended or controlled-release delivery system will result in the uncontrolled delivery of oxycodone and pose a significant risk to the abuser that could result in overdose and death [see Warnings and Precautions (5.1)]. The risk of fatal overdose is further increased when oxycodone is abused concurrently with alcohol or other CNS depressants, including other opioids [see Warnings and Precautions (5.3)]. Abuse may occur by taking intact tablets without legitimate purpose, by crushing and chewing or snorting the crushed formulation, or by injecting a solution made from the crushed formulation.
Drug addiction is characterized by compulsive abuse, repeated use for non-medical purposes, loss of control over intake, craving of psychic effects and continued abuse despite harm or risk of harm in medical, social, legal or occupational domains. There is a potential for drug addiction to develop following exposure to opioids, including oxycodone. Drug addiction is a treatable disease, but relapse is common.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of addiction and is characterized by intentional misuse for non-medical purposes, often in combination with other psychoactive substances. OxyContin has been diverted for non-medical use.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, proper dispensing and correct storage and handling are appropriate measures that help to limit misuse and abuse of opioid drugs. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
9.3 Dependence
Physical dependence to an opioid is manifested by characteristic withdrawal signs and symptoms after abrupt discontinuation of a drug, significant dose reduction or upon administration of an antagonist. Physical dependence and tolerance are not unusual during chronic opioid therapy.
The opioid abstinence or withdrawal syndrome in adults is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, piloerection, myalgia, mydriasis, irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. [See Use In Specific Populations (8.2)]
In general, opioids should not be abruptly discontinued [see Dosage and Administration (2.9)].
-
10 OVERDOSAGE
Acute overdosage with OxyContin can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, hypotension, partial or complete airway obstruction, atypical snoring and death.
It is important to take the pharmacokinetic profile of OxyContin into account when treating overdose. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects as opioid continues to be absorbed from ingested tablets.
Deaths due to overdose have been reported with abuse and misuse of whole OxyContin tablets, and with abuse and misuse by ingesting, inhaling, or injecting crushed tablets. Review of case reports has indicated that the risk of fatal overdose is further increased when OxyContin is abused concurrently with alcohol or other CNS depressants, including other opioids.
In the treatment of OxyContin overdosage, primary attention should be given to the maintenance of a patent airway, and of effective ventilation (clearance of CO2) and oxygenation, whether by spontaneous, assisted or controlled respiration. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
The pure opioid antagonists such as naloxone or nalmefene are specific antidotes against respiratory depression from opioid overdose. Since the duration of action of OxyContin may exceed that of the antagonist, especially when the overdose involves intact tablets, keep the patient under continued surveillance and administer repeated doses of the antagonist according to the antagonist labeling as needed to maintain adequate respiration. Do not administer opioid antagonists in the absence of clinically significant respiratory or circulatory depression secondary to oxycodone overdose. In patients who are physically dependent on any opioid agonist including OxyContin, an abrupt partial or complete reversal of opioid effects may precipitate an acute abstinence (or withdrawal) syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. See the prescribing information for the specific opioid antagonist for details of its proper use.
-
11 DESCRIPTION
OxyContin (oxycodone hydrochloride controlled-release) is an opioid analgesic supplied in 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg and 80 mg tablets for oral administration. The tablet strengths describe the amount of oxycodone per tablet as the hydrochloride salt. The structural formula for oxycodone hydrochloride is as follows:
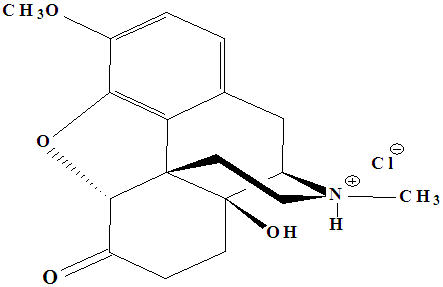
C18 H21 NO4 HCl MW 351.83
The chemical name is 4, 5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride.
Oxycodone is a white, odorless crystalline powder derived from the opium alkaloid, thebaine. Oxycodone hydrochloride dissolves in water (1 g in 6 to 7 mL). It is slightly soluble in alcohol (octanol water partition coefficient 0.7).
The 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg and 80 mg tablets contain the following inactive ingredients: butylated hydroxytoluene (BHT), hypromellose, polyethylene glycol 400, polyethylene oxide, magnesium stearate, titanium dioxide.
The 10 mg tablets also contain: hydroxypropyl cellulose.
The 15 mg tablets also contain: black iron oxide, yellow iron oxide, and red iron oxide.
The 20 mg tablets also contain: polysorbate 80 and red iron oxide.
The 30 mg tablets also contain: polysorbate 80, red iron oxide, yellow iron oxide, and black iron oxide.
The 40 mg tablets also contain: polysorbate 80 and yellow iron oxide.
The 60 mg tablets also contain: polysorbate 80, red iron oxide and black iron oxide.
The 80 mg tablets also contain: hydroxypropyl cellulose, yellow iron oxide and FD&C Blue #2/Indigo Carmine Aluminum Lake.
-
12 CLINICAL PHARMACOLOGY
Oxycodone is a pure mu receptor opioid agonist whose principal therapeutic action is analgesia. Other members of the class known as opioid agonists include substances such as morphine, hydromorphone, fentanyl, codeine, hydrocodone and oxymorphone. Pharmacological effects of opioid agonists include anxiolysis, euphoria, feelings of relaxation, respiratory depression, constipation, miosis, and cough suppression, as well as analgesia. Increasing doses of pure mu receptor agonists are associated with increasing analgesia. There is no defined maximum dose; the ceiling to analgesic effectiveness is imposed only by adverse reactions, the more serious of which may include somnolence and respiratory depression.
12.1 Mechanism of Action
Central Nervous System
The precise mechanism of the analgesic action is unknown. However, specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and are thought to play a role in the analgesic effects of this drug.
12.2 Pharmacodynamics
A single-dose, double-blind, placebo- and dose-controlled study was conducted using OxyContin (10, 20, and 30 mg) in an analgesic pain model involving 182 patients with moderate to severe pain. OxyContin doses of 20 mg and 30 mg produced statistically significant pain reduction compared to placebo.
Central Nervous System
Oxycodone produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves both a reduction in the responsiveness of the brain stem respiratory centers to increases in CO2 tension and to electrical stimulation.
Oxycodone depresses the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia.
Oxycodone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in the setting of oxycodone overdose [See Overdosage (10)].
Gastrointestinal Tract and Other Smooth Muscle
Oxycodone causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in gastric, biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Cardiovascular System
Oxycodone may produce release of histamine with or without associated peripheral vasodilation. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.
Endocrine System
Opioids may influence the hypothalamic-pituitary-adrenal or -gonadal axes. Some changes that can be seen include an increase in serum prolactin, and decreases in plasma cortisol and testosterone. Clinical signs and symptoms may be manifest from these hormonal changes.
Immune System
In vitro and animal studies indicate that opioids have a variety of effects on immune functions, depending on the context in which they are used. The clinical significance of these findings is unknown.
Concentration – Efficacy Relationships
Studies in normal volunteers and patients reveal predictable relationships between oxycodone dosage and plasma oxycodone concentrations, as well as between concentration and certain expected opioid effects, such as pupillary constriction, sedation, overall subjective "drug effect", analgesia and feelings of "relaxation".
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with potent agonist opioids. As a result, patients must be treated with individualized titration of dosage to the desired effect. The minimum effective analgesic concentration of oxycodone for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome and/or the development of analgesic tolerance.
Concentration – Adverse Reaction Relationships
There is a relationship between increasing oxycodone plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related side effects.
The dose of OxyContin must be individualized [see Dosage and Administration (2.6)], because the effective analgesic dose for some patients will be too high to be tolerated by other patients.
12.3 Pharmacokinetics
The activity of OxyContin is primarily due to the parent drug oxycodone. OxyContin is designed to provide delivery of oxycodone over 12 hours.
Cutting, breaking, chewing, crushing or dissolving OxyContin impairs the controlled-release delivery mechanism and results in the rapid release and absorption of a potentially fatal dose of oxycodone.
Oxycodone release from OxyContin is pH independent. The oral bioavailability of oxycodone is 60% to 87%. The relative oral bioavailability of oxycodone from OxyContin to that from immediate-release oral dosage forms is 100%. Upon repeated dosing with OxyContin in healthy subjects in pharmacokinetic studies, steady-state levels were achieved within 24-36 hours. Oxycodone is extensively metabolized and eliminated primarily in the urine as both conjugated and unconjugated metabolites. The apparent elimination half-life of oxycodone following the administration of OxyContin was 4.5 hours compared to 3.2 hours for immediate-release oxycodone.
Absorption
About 60% to 87% of an oral dose of oxycodone reaches the central compartment in comparison to a parenteral dose. This high oral bioavailability is due to low pre-systemic and/or first-pass metabolism.
Plasma Oxycodone Concentration Over Time
Dose proportionality has been established for OxyContin 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg, and 80 mg tablet strengths for both peak plasma concentrations (Cmax) and extent of absorption (AUC) (see Table 3). Given the short elimination half-life of oxycodone, steady-state plasma concentrations of oxycodone are achieved within 24-36 hours of initiation of dosing with OxyContin. In a study comparing 10 mg of OxyContin every 12 hours to 5 mg of immediate-release oxycodone every 6 hours, the two treatments were found to be equivalent for AUC and Cmax, and similar for Cmin (trough) concentrations.
TABLE 3 Mean [% coefficient of variation] Regimen Dosage Form AUC
(nghr/mL) *Cmax
(ng/mL)Tmax
(hr)* for single-dose AUC = AUC0-inf †data obtained while subjects received naltrexone which can enhance absorption Single
Dose†10 mg 136 [27] 11.5 [27] 5.11 [21] 15 mg 196 [28] 16.8 [29] 4.59 [19] 20 mg 248 [25] 22.7 [25] 4.63 [22] 30 mg 377 [24] 34.6 [21] 4.61 [19] 40 mg 497 [27] 47.4 [30] 4.40 [22] 60 mg 705 [22] 64.6 [24] 4.15 [26] 80 mg 908 [21] 87.1 [29] 4.27 [26] Food Effects
Food has no significant effect on the extent of absorption of oxycodone from OxyContin.
Distribution
Following intravenous administration, the steady-state volume of distribution (Vss) for oxycodone was 2.6 L/kg. Oxycodone binding to plasma protein at 37°C and a pH of 7.4 was about 45%. Once absorbed, oxycodone is distributed to skeletal muscle, liver, intestinal tract, lungs, spleen, and brain. Oxycodone has been found in breast milk [see Use In Specific Populations (8.3)].
Metabolism
Oxycodone is extensively metabolized by multiple metabolic pathways to produce noroxycodone, oxymorphone and noroxymorphone, which are subsequently glucuronidated. Noroxycodone and noroxymorphone are the major circulating metabolites. CYP3A mediated N-demethylation to noroxycodone is the primary metabolic pathway of oxycodone with a lower contribution from CYP2D6 mediated O-demethylation to oxymorphone. Therefore, the formation of these and related metabolites can, in theory, be affected by other drugs (see Drug-Drug Interactions).
Noroxycodone exhibits very weak anti-nociceptive potency compared to oxycodone, however, it undergoes further oxidation to produce noroxymorphone, which is active at opioid receptors. Although noroxymorphone is an active metabolite and present at relatively high concentrations in circulation, it does not appear to cross the blood-brain barrier to a significant extent. Oxymorphone is present in the plasma only at low concentrations and undergoes further metabolism to form its glucuronide and noroxymorphone. Oxymorphone has been shown to be active and possessing analgesic activity but its contribution to analgesia following oxycodone administration is thought to be clinically insignificant. Other metabolites (α- and ß-oxycodol, noroxycodol and oxymorphol) may be present at very low concentrations and demonstrate limited penetration in to the brain as compared to oxycodone. The enzymes responsible for keto-reduction and glucuronidation pathways in oxycodone metabolism have not been established.
Excretion
Oxycodone and its metabolites are excreted primarily via the kidney. The amounts measured in the urine have been reported as follows: free and conjugated oxycodone 8.9%, free noroxycodone 23%, free oxymorphone less than 1%, conjugated oxymorphone 10%, free and conjugated noroxymorphone 14%, reduced free and conjugated metabolites up to 18%. The total plasma clearance was approximately 1.4 L/min in adults.
Special Populations
Elderly (≥65 years)
The plasma concentrations of oxycodone are only nominally affected by age, being 15% greater in elderly as compared to young subjects (age 21-45).
Gender
Across individual pharmacokinetic studies, average plasma oxycodone concentrations for female subjects were up to 25% higher than for male subjects on a body weight adjusted basis. The reason for this difference is unknown [see Use In Specific Populations (8.8)].
Renal Impairment
Data from a pharmacokinetic study involving 13 patients with mild to severe renal dysfunction (creatinine clearance <60 mL/min) showed peak plasma oxycodone and noroxycodone concentrations 50% and 20% higher, respectively, and AUC values for oxycodone, noroxycodone, and oxymorphone 60%, 50%, and 40% higher than normal subjects, respectively. This was accompanied by an increase in sedation but not by differences in respiratory rate, pupillary constriction, or several other measures of drug effect. There was an increase in mean elimination t½ for oxycodone of 1 hour.
Hepatic Impairment
Data from a study involving 24 patients with mild to moderate hepatic dysfunction show peak plasma oxycodone and noroxycodone concentrations 50% and 20% higher, respectively, than healthy subjects. AUC values are 95% and 65% higher, respectively. Oxymorphone peak plasma concentrations and AUC values are lower by 30% and 40%. These differences are accompanied by increases in some, but not other, drug effects. The mean elimination t½ for oxycodone increased by 2.3 hours.
Drug-Drug Interactions
Oxycodone is extensively metabolized by multiple metabolic pathways. CYP3A4 is the major enzyme involved in noroxycodone formation followed by CYP2B6, CYP2C9/19 and CYP2D6. Drugs that inhibit CYP3A4 activity, such as macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), and protease inhibitors (e.g., ritonavir), may cause decreased clearance of oxycodone which could lead to an increase in oxycodone plasma concentrations. For example, a published study showed that the co-administration of the antifungal drug, voriconazole, increased oxycodone AUC and Cmax by 3.6 and 1.7 fold, respectively. Similarly, CYP450 inducers, such as rifampin, carbamazepine, and phenytoin, may induce the metabolism of oxycodone and, therefore, may cause increased clearance of the drug which could lead to a decrease in oxycodone plasma concentrations, lack of efficacy or, possibly, development of an abstinence syndrome in a patient who had developed physical dependence to oxycodone. A published study showed that the co-administration of rifampin, a drug metabolizing enzyme inducer, decreased oxycodone (oral) AUC and Cmax by 86% and 63%, respectively.
Oxymorphone is a minor metabolite, its formation is catalyzed primarily by CYP2D6 and to a small extent by CYP2C19. The formation of oxymorphone may be blocked by a variety of drugs (such as antipsychotics, beta blockers, antidepressants, etc.) that inhibit these enzymes. However, in a study involving ten subjects using quinidine, a known inhibitor of CYP2D6, the pharmacodynamic effects of oxycodone were unchanged. The genetic expression of CYP2D6 may have some influence in the pharmacokinetic properties of oxycodone.
The in vitro drug-drug interaction studies with noroxymorphone using human liver microsomes showed no significant inhibition of CYP2D6 and CYP3A4 activities which suggests that noroxymorphone may not alter the metabolism of other drugs that are metabolized by CYP2D6 and CYP3A4, and such blockade has not been shown to be of clinical significance with oxycodone. [see Drug Interactions (7.2)]
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No animal studies to evaluate the carcinogenic potential of oxycodone have been conducted.
Mutagenesis
Oxycodone was genotoxic in the mouse lymphoma assay at concentrations of 50 mcg/mL or greater with metabolic activation and at 400 mcg/mL or greater without metabolic activation. Clastogenicity was observed with oxycodone in the presence of metabolic activation in one chromosomal aberration assay in human lymphocytes at concentrations greater than or equal to 1250 mcg/mL at 24 but not 48 hours of exposure. In a second chromosomal aberration assay with human lymphocytes, no structural clastogenicity was observed either with or without metabolic activation; however, in the absence of metabolic activation, oxycodone increased numerical chromosomal aberrations (polyploidy). Oxycodone was not genotoxic in the following assays: Ames S. typhimurium and E. coli test with and without metabolic activation at concentrations up to 5000 µg/plate, chromosomal aberration test in human lymphocytes (in the absence of metabolic activation) at concentrations up to 1500 µg/mL, and with activation after 48 hours of exposure at concentrations up to 5000 µg/mL, and in the in vivo bone marrow micronucleus assay in mice (at plasma levels up to 48 µg/mL).
Impairment of Fertility
In a study of reproductive performance, rats were administered a once daily gavage dose of the vehicle or oxycodone hydrochloride (0.5, 2, and 8 mg/kg). Male rats were dosed for 28 days before cohabitation with females, during the cohabitation and until necropsy (2-3 weeks post-cohabitation). Females were dosed for 14 days before cohabitation with males, during cohabitation and up to gestation day 6. Oxycodone hydrochloride did not affect reproductive function in male or female rats at any dose tested (≤8 mg/kg/day).
-
14 CLINICAL STUDIES
A double-blind, placebo-controlled, fixed-dose, parallel group, two-week study was conducted in 133 patients with persistent, moderate to severe pain, who were judged as having inadequate pain control with their current therapy. In this study, OxyContin 20 mg, but not 10 mg, was statistically significant in pain reduction compared with placebo.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
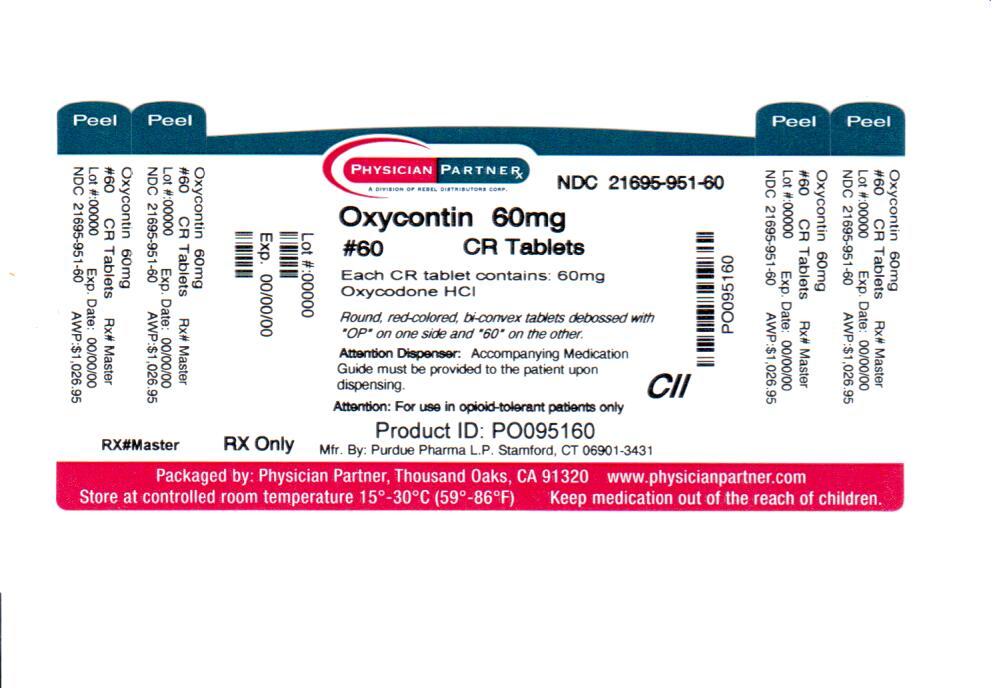
OxyContin (oxycodone hydrochloride controlled-release) Tablets 60 mg are round, red-colored, bi-convex tablets debossed with OP on one side and 60 on the other and are supplied as child-resistant closure, opaque plastic bottles of 60 (NDC: 21695-951-60).
Store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F).
Dispense in tight, light-resistant container.
-
17 PATIENT COUNSELING INFORMATION
See MEDICATION GUIDE as appended at the end of the full prescribing information
17.1 Information for Patients and Caregivers
Provide the following information to patients receiving OxyContin or their caregivers:
- Advise patients that OxyContin contains oxycodone, which is a morphine-like substance.
- Advise patients that OxyContin is designed to work properly only if swallowed whole. Taking cut, broken, chewed, crushed, or dissolved OxyContin Tablets can result in a fatal overdose.
- Advise patients that OxyContin tablets should be taken one tablet at a time.
- Advise patients not to pre-soak, lick or otherwise wet the tablet prior to placing in the mouth.
- Advise patients to take each tablet with enough water to ensure complete swallowing immediately after placing in the mouth.
- Advise patients to report adverse experiences, and episodes of increased or incident pain occurring during therapy. Individualization of dosage is essential to make optimal use of this medication.
- Advise patients not to adjust the dose of OxyContin without consulting the prescribing professional.
- Advise patients that OxyContin may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating heavy machinery).
- Advise patients not to combine OxyContin with alcohol or other central nervous system depressants (e.g. sedatives, hypnotics) except by the orders of the prescribing physician, because dangerous additive effects may occur, resulting in serious injury or death.
- Advise women of childbearing potential who become, or are planning to become, pregnant to consult their physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
- Advise patients that OxyContin is a drug with known abuse potential. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
- Advise patients that if they have been receiving treatment with OxyContin for more than a few weeks and cessation of therapy is indicated, it may be appropriate to taper the OxyContin dose, rather than abruptly discontinue it, due to the risk of precipitating withdrawal symptoms. If tapering is appropriate, their prescriber can provide a dose schedule to gradually discontinue the medication.
- Advise patients to keep OxyContin in a secure place out of the reach of children. When OxyContin is no longer needed, the unused tablets should be destroyed by flushing down the toilet.
Healthcare professionals can telephone Purdue Pharma's Medical Services Department (1-888-726-7535) for information on this product.
CAUTION
DEA Order Form Required.©2010, Purdue Pharma L.P.
Purdue Pharma L.P.
Stamford, CT 06901-3431U.S. Patent Numbers 5,508,042; 6,488,963; 7,129,248; 7,674,799; 7,674,800 and 7,683,072
301734-0D
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
-
MEDICATION GUIDE
MEDICATION GUIDE
OXYCONTIN® (ox-e-KON-tin) CII
(oxycodone hydrochloride controlled-release)
TabletsRead this Medication Guide before you start taking OxyContin and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about OxyContin?
- OxyContin can cause serious side effects, including addiction or death.
- Do not cut, break, chew, crush, or dissolve OxyContin before swallowing. If OxyContin is taken in this way, the medicine in the tablets will be released too fast. This is dangerous. It may cause you to stop breathing, and may lead to death.
- OxyContin is not for use to treat pain that you only have once in a while ("as needed").
- Do not take OxyContin 60 mg or 80 mg tablets unless you are "opioid tolerant." Opioid tolerant means that you regularly use OxyContin or another opioid medicine for your constant (around-the-clock) pain and your body is used to it.
- Do not take more than 40 mg of OxyContin in one dose or more than 80 mg of OxyContin in one day unless you are "opioid tolerant." This may cause you to stop breathing and may lead to death.
- OxyContin is a federally controlled substance (CII) because it is a strong opioid pain medicine that can be abused by people who abuse prescription medicines or street drugs.
- Prevent theft, misuse and abuse. Keep OxyContin in a safe place, to keep it from being stolen. OxyContin can be a target for people who misuse or abuse prescription medicines or street drugs.
- Never give OxyContin to anyone else, even if they have the same symptoms you have. It may harm them and even cause death.
- Before taking OxyContin, tell your doctor if you or a family member have been addicted to or abused other medicines, street drugs, or alcohol, or if you have a history of mental illness.
- Do not drink alcohol while using OxyContin. Using alcohol with OxyContin may increase your risk of dangerous side effects, including death.
- Certain medicines can interact with OxyContin and cause you to have high levels of oxycodone in your blood. This may cause you to stop breathing and lead to death. Before taking OxyContin, tell your healthcare provider if you take an antibiotic, an antifungal medicine, or an anti-HIV medicine.
What is OxyContin?
- OxyContin is a prescription medicine used when an opioid medicine is needed to manage moderate to severe pain that continues around-the-clock and is expected to last for a long period of time.
- It is not known if OxyContin is safe and effective in children younger than 18 years.
- OxyContin is not for use:
- to manage pain "as needed"
- before surgery to manage any pain from your surgery
- to manage pain after surgery if the pain is mild and is not expected to last for a long period of time
- If you already take OxyContin, it may be used to manage your pain after surgery if:
- it has been at least 12 to 24 hours after your surgery, and
- your pain from surgery is expected to be moderate to severe, and last for a long period of time.
Who should not take OxyContin?
Do not take OxyContin if you:
- are allergic to any of its ingredients. See the end of this Medication Guide for a list of the ingredients in OxyContin.
- have had a severe allergic reaction to a medicine that contains oxycodone. Ask your healthcare provider if you are not sure.
- are having an asthma attack or have severe asthma, trouble breathing, or lung problems
- have a bowel blockage called paralytic ileus
What should I tell my healthcare provider before taking OxyContin?
OxyContin may not be right for you. Before taking OxyContin, tell your doctor if you:
- have trouble breathing or lung problems
- have had a head injury
- have liver or kidney problems
- have adrenal gland problems, such as Addison's disease
- have severe scoliosis that affects your breathing
- have thyroid problems
- have enlargement of your prostate or a urethral stricture
- have or had convulsions or seizures
- have a past or present drinking problem or alcoholism
- have hallucinations or other severe mental problems
- have past or present substance abuse or drug addiction
- have any other medical conditions
- are pregnant or plan to become pregnant. If you take OxyContin regularly before your baby is born, your newborn baby may have signs of withdrawal because their body has become used to the medicine. Signs of withdrawal in a newborn baby can include:
- irritability
- crying more than usual
- shaking (tremors)
- jitteriness
- breathing faster than normal
- diarrhea or more stools than normal
- sneezing
- yawning
- vomiting
- fever
If you take OxyContin right before your baby is born, your baby could have breathing problems at birth.
- are breast-feeding. You should not take OxyContin if you are nursing. Some oxycodone from OxyContin passes into breast milk. A nursing baby could become very drowsy or have difficulty breathing or feeding well.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Sometimes the doses of medicines that you take with OxyContin may need to be changed if used together.
- See "What is the most important information I should know about OxyContin?"
- Be especially careful about taking other medicines that make you sleepy such as:
- pain medicines
- sleeping pills
- anxiety medicines
- antihistamines
- anti-depressants
- tranquilizers
- anti-nausea medicine
Do not take other medicines without talking to your healthcare provider. Your healthcare provider will tell you if it is safe to take other medicines while you take OxyContin.
Know the medicines you take. Keep a list of your medicines to show your healthcare provider and pharmacist.
How should I take OxyContin?
- See "What is the most important information I should know about OxyContin?"
- Take OxyContin exactly as prescribed. Do not change your dose unless your healthcare provider tells you to.
- Swallow OxyContin tablets whole. Do not cut, break, chew, crush, or dissolve the tablets.
-
In order to reduce the possibility of choking on the tablets or having difficulty swallowing the tablets:.
- OxyContin tablets should be taken one tablet at a time.
- Do not pre-soak, lick or otherwise wet the tablet prior to placing in your mouth.
- Take each tablet with enough water to ensure complete swallowing immediately after placing in your mouth.
- Take OxyContin every 12 hours.
- You can take OxyContin with or without food.
- If you miss a dose, take it as soon as possible. Take your next dose 12 hours later. Do not take more than your prescribed dose of OxyContin. Call your healthcare provider if you are not sure about your dose of OxyContin or when to take it.
- If you take more OxyContin than prescribed, or overdose, call your local emergency number (such as 911) or your local Poison Control Center right away, or get emergency help.
- Talk with your healthcare provider regularly about your pain to see if you still need to take OxyContin.
What should I avoid while taking OxyContin?
- Do not drink alcohol while using OxyContin. See "What is the most important information I should know about OxyContin?" Do not drive, operate heavy machinery, or do other dangerous activities, especially when you start taking OxyContin and when your dose is changed, until you know how you react to this medicine. OxyContin can make you sleepy, and also cause you to feel dizzy. Ask your healthcare provider to tell you when it is okay to do these activities.
What are the possible side effects of OxyContin?
OxyContin can cause serious side effects, including:
- See "What is the most important information I should know about OxyContin?"
-
OxyContin can cause serious breathing problems that can become life-threatening, especially if OxyContin is used the wrong way. Call your healthcare provider or get medical help right away if:
- your breathing slows down
- you have shallow breathing (little chest movement with breathing)
- you feel faint, dizzy, confused, or
- you have any other unusual symptoms
These can be signs or symptoms that you have taken too much OxyContin (overdose) or the dose is too high for you. These symptoms may lead to serious problems or death if not treated right away.
- Central nervous system effects, including sleepiness, dizziness, passing out, becoming unconscious, or coma.
- OxyContin may cause a worsening of seizures in people who already have seizures.
- OxyContin can cause your blood pressure to drop. This can make you feel dizzy and faint if you get up too fast from sitting or lying down. Low blood pressure is also more likely to happen if you take other medicines that can also lower your blood pressure. Severe low blood pressure can happen if you lost blood or take certain other medicines.
-
OxyContin can cause physical dependence. Do not stop taking OxyContin or any other opioid without talking to your healthcare provider about how to slowly stop your medicine. You could become sick with uncomfortable withdrawal symptoms because your body has become used to these medicines. Physical dependence is not the same as drug addiction. Tell your healthcare provider if you have any of these signs or symptoms of withdrawal while slowly stopping OxyContin:
- feel restless
- tearing eyes
- runny nose
- yawning
- sweating
- chills or hair on your arms "standing up"
- muscle aches, backache
- dilated pupils of your eyes
- feel irritable or anxious
- nausea, loss of appetite, vomiting, diarrhea
- increase in your blood pressure, breathing faster, or your heart beats faster
- There is a chance of abuse or addiction with OxyContin. The chance is higher if you are or have been addicted to or abused other medicines, street drugs, or alcohol, or if you have a history of mental problems.
The most common side effects of OxyContin include:
- constipation
- nausea
- drowsiness
- dizziness
- itching
- vomiting
- headache
- dry mouth
- weakness
- sweating
Some of these side effects may decrease with continued use. Talk with your healthcare provider if you continue to have these side effects. These are not all the possible side effects of OxyContin. For a complete list, ask your healthcare provider or pharmacist.
Constipation (not often enough or hard bowel movements) is a very common side effect of pain medicines (opioids) including OxyContin, and is unlikely to go away without treatment. Talk to your healthcare provider about dietary changes, and the use of laxatives (medicines to treat constipation) and stool softeners to prevent or treat constipation while taking OxyContin.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1–800–FDA–1088.
How should I store OxyContin?
- Keep OxyContin out of the reach of children. Accidental overdose by a child is dangerous and can lead to death.
- Store OxyContin at 59° F to 86°F (15° C to 30° C)
- Keep OxyContin in the container it comes in.
- Keep the container tightly closed and away from light.
- After you stop taking OxyContin, flush the unused tablets down the toilet.
General information about OxyContin
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use OxyContin for a condition for which it was not prescribed. Never give your OxyContin to other people even if they have the same symptoms you have.Selling or giving away OxyContin may harm others, even causing death, and is against the law.
This Medication Guide summarizes the most important information about OxyContin. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about OxyContin that is written for health professionals. For more information about OxyContin, go to www.purduepharma.com or call 1-888-726-7535.
What are the ingredients of OxyContin?
Active ingredient: oxycodone hydrochloride
Inactive ingredients in all strengths: butylated hydroxytoluene (BHT), hypromellose, polyethylene glycol 400, polyethylene oxide, magnesium stearate, titanium dioxide- The 10 mg tablets also contain: hydroxypropyl cellulose.
- The 15 mg tablets also contain: black iron oxide, yellow iron oxide, and red iron oxide.
- The 20 mg tablets also contain: polysorbate 80 and red iron oxide.
- The 30 mg tablets also contain: polysorbate 80, red iron oxide, yellow iron oxide, and black iron oxide.
- The 40 mg tablets also contain: polysorbate 80 and yellow iron oxide.
- The 60 mg tablets also contain: polysorbate 80, red iron oxide and black iron oxide.
- The 80 mg tablets also contain: hydroxypropyl cellulose, yellow iron oxide and FD&C Blue #2/Indigo Carmine Aluminum Lake.
Always check to make sure that the medicine you are taking is the correct one. The dosage strength and appearance of each OxyContin tablet are as follows:
- 10 mg: white-colored with "OP" on one side and "10" on the other
- 15 mg: gray-colored with "OP" on one side and "15" on the other
- 20 mg: pink-colored with "OP" on one side and "20" on the other
- 30 mg: brown-colored with "OP" on one side and "30" on the other
- 40 mg: yellow-colored with "OP" on one side and "40" on the other
- 60 mg: red-colored with "OP" on one side and "60" on the other
- 80 mg: green-colored with "OP" on one side and "80" on the other
This Medication Guide has been approved by the U.S. Food and Drug Administration.
CAUTION
DEA Order Form Required.©2010, Purdue Pharma L.P.
Purdue Pharma L.P.
Stamford, CT 06901-3431U.S. Patent Numbers 5,508,042; 6,488,963; 7,129,248; 7,674,799; 7,674,800 and 7,683,072
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
OXYCONTIN
oxycodone hydrochloride tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-951(NDC:59011-460) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength oxycodone hydrochloride (UNII: C1ENJ2TE6C) (oxycodone - UNII:CD35PMG570) oxycodone hydrochloride 60 mg Inactive Ingredients Ingredient Name Strength butylated hydroxytoluene (UNII: 1P9D0Z171K) hypromelloses (UNII: 3NXW29V3WO) polyethylene glycol 400 (UNII: B697894SGQ) polyethylene glycol (UNII: 3WJQ0SDW1A) magnesium stearate (UNII: 70097M6I30) titanium dioxide (UNII: 15FIX9V2JP) polysorbate 80 (UNII: 6OZP39ZG8H) ferric oxide red (UNII: 1K09F3G675) ferrosoferric oxide (UNII: XM0M87F357) Product Characteristics Color RED Score no score Shape ROUND Size 10mm Flavor Imprint Code 60;OP Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-951-60 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022272 08/08/2010 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK
Trademark Results [OxyContin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OXYCONTIN 90470025 not registered Live/Pending |
Yangzhou Zhixing Network Technology Co., Ltd. 2021-01-15 |
 OXYCONTIN 75069553 2033914 Live/Registered |
PURDUE PHARMA L.P. 1996-03-08 |
 OXYCONTIN 74339006 2004586 Live/Registered |
PURDUE PHARMA L.P. 1992-12-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.