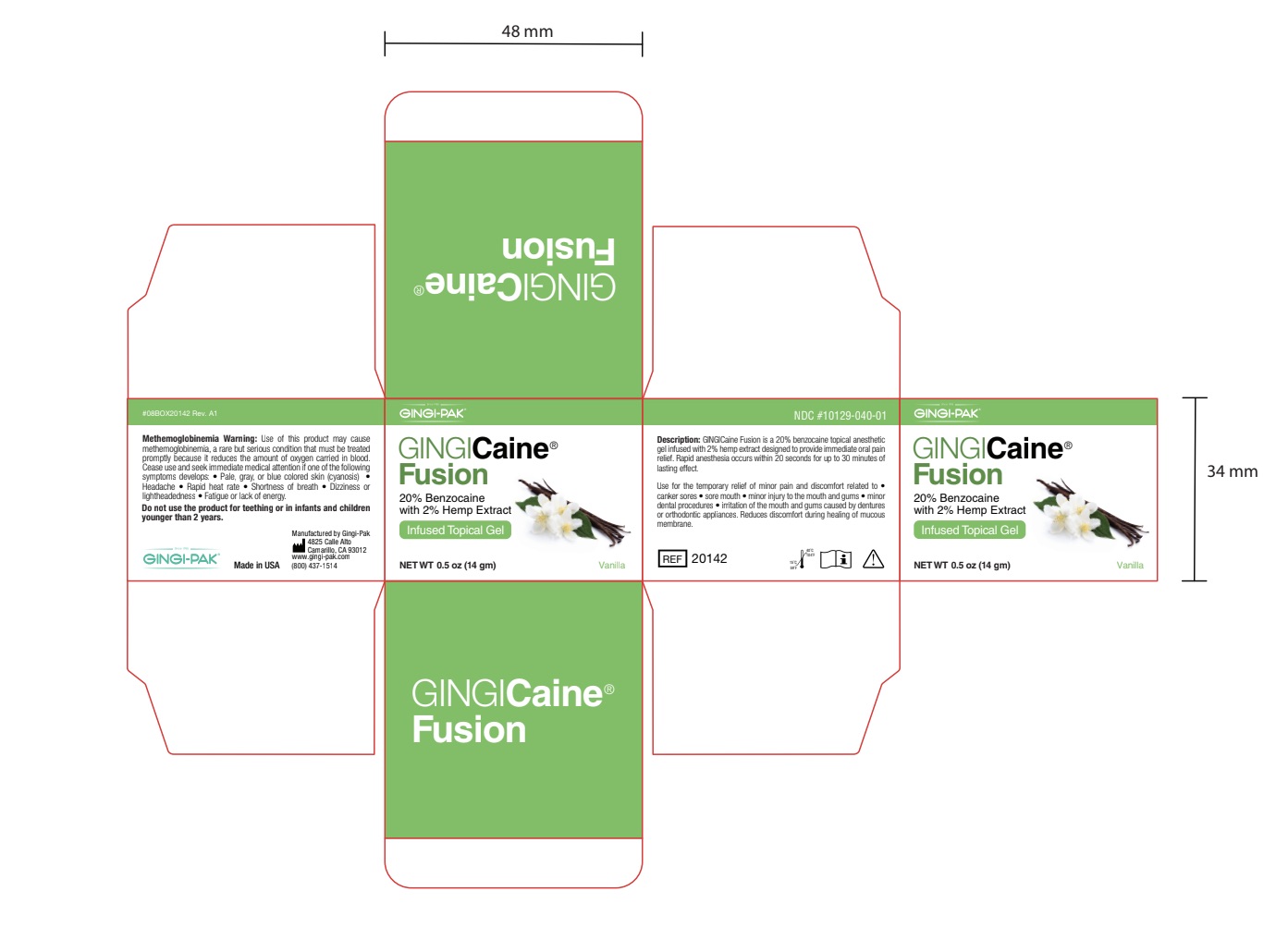
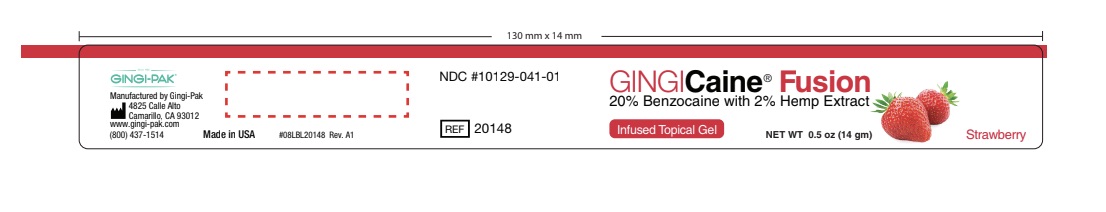
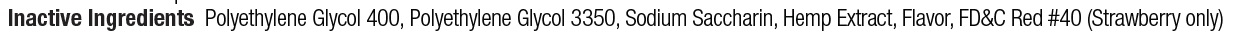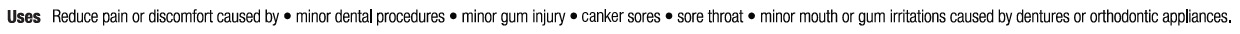GINGICAINE FUSION- benzocaine gel
GingiCaine Fusion by
Drug Labeling and Warnings
GingiCaine Fusion by is a Otc medication manufactured, distributed, or labeled by Gingi-Pak a Division of the Belport, Jeff Nichols. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Allergy Alert
- Consult a doctor
- Avoid Excessive heat
- Caution
- Contraindications
- Directions for Use
- Dosage & Administration
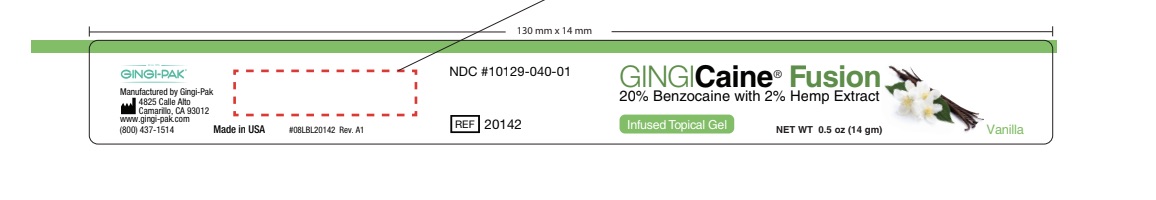
- Inactive Ingredients
- Indications
- Keep out of reach of children
- Overdosage
- Precautions
- Temper Evident Statement
- Uses
- Warnings
- Description
- Principal Display
-
INGREDIENTS AND APPEARANCE
GINGICAINE FUSION
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10129-041 Route of Administration TOPICAL, DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 20 g in 100 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) 24 g in 100 g CANNABIDIOL (UNII: 19GBJ60SN5) 2 g in 100 g POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 52 g in 100 g POTASSIUM SODIUM SACCHARATE (UNII: 73U34YC90U) 2 g in 100 g Product Characteristics Color Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10129-041-01 14 g in 1 JAR; Type 0: Not a Combination Product 06/04/2021 2 NDC: 10129-041-20 20 in 1 BOX 06/04/2021 2 1.2 g in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/04/2021 GINGICAINE FUSION
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10129-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 20 g in 100 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) 24 g in 100 g CANNABIDIOL (UNII: 19GBJ60SN5) 2 g in 100 g POTASSIUM SODIUM SACCHARATE (UNII: 73U34YC90U) 2 g in 100 g POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 52 g in 100 g Product Characteristics Color Score Shape Size Flavor VANILLA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10129-040-01 14 g in 1 JAR; Type 0: Not a Combination Product 06/04/2021 2 NDC: 10129-040-20 20 in 1 BOX 06/04/2021 2 1.2 g in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/04/2021 Labeler - Gingi-Pak a Division of the Belport (008480121) Registrant - Jeff Nichols (008480121) Establishment Name Address ID/FEI Business Operations Gingi-Pak a Division of the Belport 008480121 manufacture(10129-040, 10129-041)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.