EYSUVIS- loteprednol etabonate suspension/ drops
Eysuvis by
Drug Labeling and Warnings
Eysuvis by is a Prescription medication manufactured, distributed, or labeled by Kala Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EYSUVIS™ safely and effectively. See full prescribing information for EYSUVIS.
EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25%,
for topical ophthalmic use
Initial U.S. Approval: 1998
INDICATIONS AND USAGE
EYSUVIS is a corticosteroid indicated for the short-term (up to two weeks) treatment of the signs and symptoms of dry eye disease. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Ophthalmic suspension containing 2.5 mg/mL of loteprednol etabonate. (3)
CONTRAINDICATIONS
EYSUVIS, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures. (4)
WARNINGS AND PRECAUTIONS
- Delayed Healing and Corneal Perforation:The initial prescription and each renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining. (5.1)
- Intraocular Pressure (IOP) Increase: Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Renewal of the medication order should be made by a physician only after examination of the patient and evaluation of the IOP.(5.2)
- Cataracts: Use of corticosteroids may result in posterior subcapsular cataract formation. (5.3)
- Bacterial Infections: Use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, corticosteroids may mask infection or enhance existing infection. (5.4)
- Viral Infections: Use of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). (5.5)
-
Fungal Infections: Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a corticosteroid has been used or is in use. (5.6)
ADVERSE REACTIONS
The most common adverse drug reaction following the use of EYSUVIS for two weeks was instillation site pain, which was reported in 5% of patients. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Alcon Laboratories, Inc. at 1-800-757-9195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Delayed Healing and Corneal Perforation
5.2 Intraocular Pressure (IOP) Increase
5.3 Cataracts
5.4 Bacterial Infections
5.5 Viral Infections
5.6 Fungal Infections
5.7 Risk of Contamination
5.8 Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
Instill one to two drops of EYSUVIS into each eye four times daily for up to two weeks. This product should only be renewed after examination under magnification such as a slit lamp and evaluation of the intraocular pressure. [see Warnings and Precautions (5.1) and (5.2)].
2.2 Administration Instructions
Instruct patient to wash hands well before each use. Shake for two to three seconds before using.
If the patient is using other eye drops in addition to EYSUVIS, advise the patient to wait at least 5 minutes between instillation of EYSUVIS and other eye drops.
If a dose is missed, take the missed dose when remembered.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
EYSUVIS, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures.
-
5 WARNINGS AND PRECAUTIONS
5.1 Delayed Healing and Corneal Perforation
Topical corticosteroids have been known to delay healing and cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation. The initial prescription and each renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining.
5.2 Intraocular Pressure (IOP) Increase
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, as well as defects in visual acuity and fields of vision. Corticosteroids should be used with caution in the presence of glaucoma. Renewal of the medication order should be made by a physician only after examination of the patient and evaluation of the IOP.
5.4 Bacterial Infections
Use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions of the eye, corticosteroids may mask infection or enhance existing infection.
5.5 Viral Infections
Use of corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex).
5.6 Fungal Infections
Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a corticosteroid has been used or is in use. Fungal cultures should be taken when appropriate.
-
6 ADVERSE REACTIONS
Adverse reactions associated with ophthalmic corticosteroids include elevated intraocular pressure, which may be associated with infrequent optic nerve damage, visual acuity and field defects, posterior subcapsular cataract formation, delayed wound healing and secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reaction observed in clinical trials with EYSUVIS was instillation site pain, which was reported in 5% of patients.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well controlled studies with loteprednol etabonate in pregnant women. Loteprednol etabonate produced teratogenicity at clinically relevant doses in the rabbit and rat when administered orally during pregnancy. Loteprednol etabonate produced malformations when administered orally to pregnant rabbits at doses 1.4 times the recommended human ophthalmic dose (RHOD) and to pregnant rats at doses 34 times the RHOD. In pregnant rats receiving oral doses of loteprednol etabonate during the period equivalent to the last trimester of pregnancy through lactation in humans, survival of offspring was reduced at doses 3.4 times the RHOD. Maternal toxicity was observed in rats at doses 347 times the RHOD, and a maternal no observed adverse effect level (NOAEL) was established at 34 times the RHOD.
The background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies.
Data
Animal Data
Embryofetal studies were conducted in pregnant rabbits administered loteprednol etabonate by oral gavage on gestation days 6 to 18, to target the period of organogenesis. Loteprednol etabonate produced fetal malformations at 0.1 mg/kg (1.4 times the recommended human ophthalmic dose (RHOD) based on body surface area, assuming 100% absorption). Spina bifida (including meningocele) was observed at 0.1 mg/kg, and exencephaly and craniofacial malformations were observed at 0.4 mg/kg (5.6 times the RHOD). At 3 mg/kg (41 times the RHOD), loteprednol etabonate was associated with increased incidences of abnormal left common carotid artery, limb flexures, umbilical hernia, scoliosis, and delayed ossification. Abortion and embryofetal lethality (resorption) occurred at 6 mg/kg (83 times the RHOD). A NOAEL for developmental toxicity was not established in this study. The NOAEL for maternal toxicity in rabbits was 3 mg/kg/day.
Embryofetal studies were conducted in pregnant rats administered loteprednol etabonate by oral gavage on gestation days 6 to 15, to target the period of organogenesis. Loteprednol etabonate produced fetal malformations, including absent innominate artery at 5 mg/kg (34 times the RHOD); and cleft palate, agnathia, cardiovascular defects, umbilical hernia, decreased fetal body weight and decreased skeletal ossification at 50 mg/kg (347 times the RHOD). Embryofetal lethality (resorption) was observed at 100 mg/kg (695 times the RHOD). The NOAEL for developmental toxicity in rats was 0.5 mg/kg (3.4 times the RHOD). Loteprednol etabonate was maternally toxic (reduced body weight gain) at 50 mg/kg/day. The NOAEL for maternal toxicity was 5 mg/kg.
A peri-/postnatal study was conducted in rats administered loteprednol etabonate by oral gavage from gestation day 15 (start of fetal period) to postnatal day 21 (the end of lactation period). At 0.5 mg/kg (3.4 times the clinical dose), reduced survival was observed in live-born offspring. Doses ≥ 5 mg/kg (34 times the RHOD) caused umbilical hernia/incomplete gastrointestinal tract. Doses ≥ 50 mg/kg (347 times the RHOD) produced maternal toxicity (reduced body weight gain, death), decreased number of live-born offspring, decreased birth weight, and delays in postnatal development. A developmental NOAEL was not established in this study. The NOAEL for maternal toxicity was 5 mg/kg.
8.2 Lactation
There are no data on the presence of loteprednol etabonate in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother's clinical need for EYSUVIS and any potential adverse effects on the breastfed infant from EYSUVIS.
-
11 DESCRIPTION
Loteprednol etabonate is a corticosteroid. Its chemical name is chloromethyl 17α-[(ethoxycarbonyl)oxy]-11β-hydroxy-3-oxoandrosta-1,4-diene-17β-carboxylate. Its molecular formula is C24H31ClO7 and its chemical structure is:
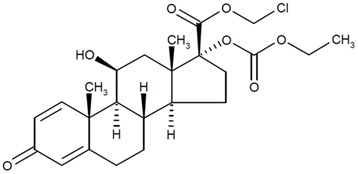
C24H31ClO7
Mol. Wt. 467.0EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25% contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. Each mL contains:
- ACTIVE: loteprednol etabonate 2.5 mg (0.25%)
- INACTIVES: glycerin, sodium citrate dihydrate, sodium chloride, Poloxamer 407, edetate disodium dihydrate, citric acid, and water for injection.
- PRESERVATIVE: benzalkonium chloride 0.01%
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids inhibit the inflammatory response to a variety of inciting agents and delay or slow healing. Corticosteroids inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation. While glucocorticoids are known to bind to and activate the glucocorticoid receptor, the molecular mechanisms involved in glucocorticoid/glucocorticoid receptor-dependent modulation of inflammation are not clearly established. However, corticosteroids are thought to inhibit prostaglandin production.
12.3 Pharmacokinetics
Loteprednol etabonate is lipid soluble and can penetrate into cells. Loteprednol etabonate is synthesized through structural modifications of prednisolone-related compounds so that it will undergo a predictable transformation to an inactive metabolite. Based upon in vivo and in vitro preclinical metabolism studies, loteprednol etabonate undergoes extensive metabolism to inactive carboxylic acid metabolites, PJ-91 and PJ-90.
Following bilateral topical ocular dosing of two drops of EYSUVIS four times a day for 14 days in 20 healthy adult subjects, the plasma concentrations of loteprednol etabonate were below the limit of quantitation (1 ng/mL) at all timepoints.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of loteprednol etabonate. Loteprednol etabonate was not genotoxic in vitro in the Ames test, the mouse lymphoma thymidine kinase (tk) assay, in a chromosome aberration test in human lymphocytes, or in vivo in the single dose mouse micronucleus assay. Treatment of male and female rats with 25 mg/kg/day of loteprednol etabonate (174 times the RHOD based on body surface area, assuming 100% absorption) prior to and during mating caused pre-implantation loss and decreased the number of live fetuses/live births. The NOAEL for fertility in rats was 5 mg/kg/day (34 times the RHOD).
-
14 CLINICAL STUDIES
The safety and efficacy of EYSUVIS for the treatment of dry eye disease was assessed in approximately 2900 patients with dry eye disease. Patients received either EYSUVIS or vehicle (1:1 ratio) four times a day for 2 weeks in 4 multi-centered, randomized, double-masked, placebo-controlled trials. The use of artificial tears was not allowed during the trials.
Effects on Symptoms of Dry Eye Disease
Ocular discomfort severity (ODS) was rated by patients daily over the course of the trial using a visual analog scale (0 = very mild, 100 = very severe). A larger reduction in ocular discomfort severity favoring EYSUVIS was observed at Day 15 in the patient population (see Figure 1).
Figure 1: Mean Change (SD) from Baseline and Treatment Difference (EYSUVIS-
Vehicle) in Ocular Discomfort Severity Score in Patients with Dry Eye Disease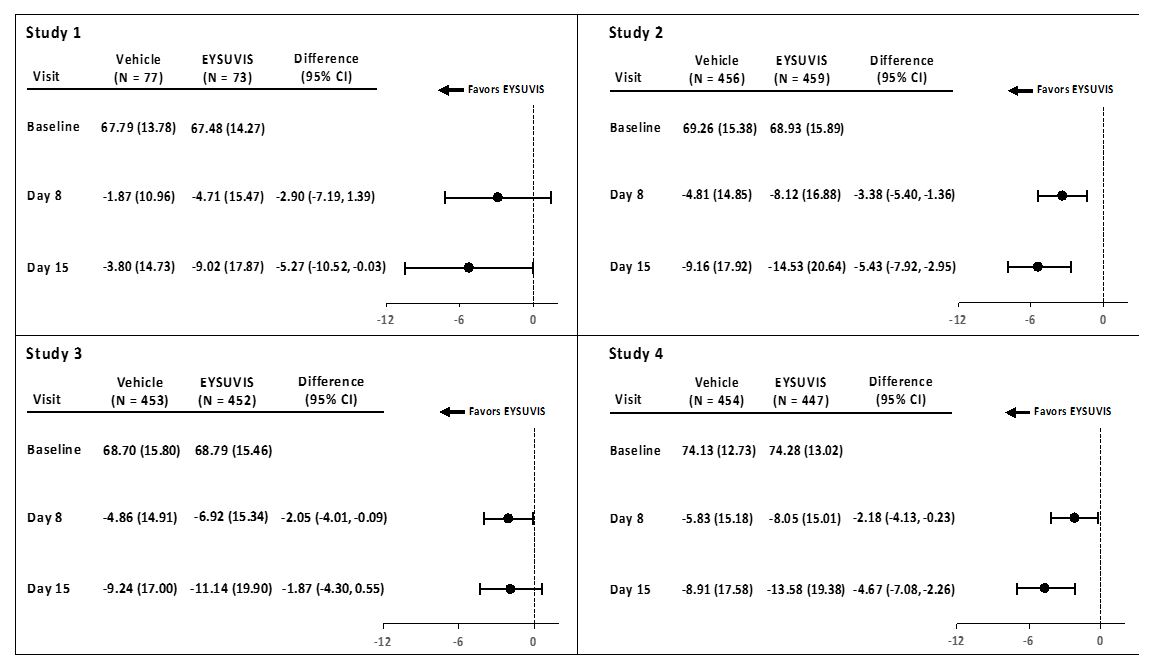
Treatment differences between the EYSUVIS and vehicle groups are displayed for each study, based on least square means and 2-sided confidence intervals for the change from baseline.
Effects on Signs of Dry Eye Disease
Conjunctival hyperemia was graded using the Cornea and Contact Lens Research Unit (CCLRU) grading scale (0 = none; 1 = very slight; 2 = slight; 3 = moderate; 4 = severe). A larger reduction in hyperemia favoring EYSUVIS was observed at Day 15 in all four trials (Figure 2).
Figure 2: Mean Change (SD) from Baseline and Treatment Difference (EYSUVIS-
Vehicle) in Conjunctival Hyperemia in Patients with Dry Eye Disease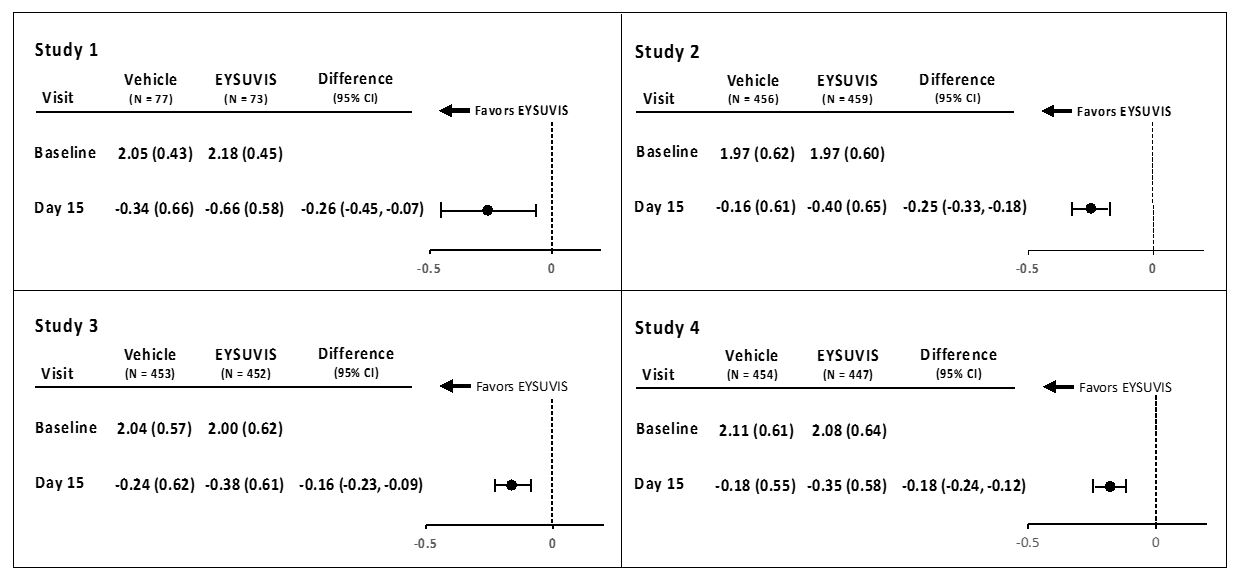
Treatment differences between the EYSUVIS and vehicle groups are displayed for each study, based on least square means and 2-sided confidence intervals for the change from baseline.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25% is a sterile ophthalmic suspension. It is supplied in a white, low-density polyethylene dropper bottle with a linear low-density polyethylene tip, a pink high-density polyethylene cap, and a white low-density polyethylene tamper-evident overcap in the following size:
8.3 mL in a 10 mL bottle (NDC: 71571-333-83)
Storage and Handling
Do not use if tamper-evident overcap seal is not intact.
The white tamper-evident overcap can be thrown away. Retain the pink cap and keep the bottle tightly closed when not in use.
Store upright at 15°C to 25°C (59°F to 77°F). Do not freeze. After opening, EYSUVIS can be used until the expiration date on the bottle.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Administration
Instruct the patient to shake the bottle for two to three seconds before using. If a dose is missed, take the missed dose when remembered.
Corneal Status and Intraocular Pressure Monitoring
The initial prescription and each renewal of the medication order should be made only after evaluation of the intraocular pressure and examination of the patient with the aid of magnification, such as slit-lamp biomicroscopy.
Risk of Contamination
Advise patients to wash their hands well before each use. Advise patients not to allow the dropper tip to touch any surface, as this may contaminate the suspension.
Risk of Secondary Infection
Advise the patient to consult a physician, if pain develops, redness, itching, or inflammation becomes aggravated.
Contact Lens Wear
Advise patients that the preservative in EYSUVIS may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of EYSUVIS and may be reinserted 15 minutes following administration.
Manufactured for:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
Part # 70016481-00
U.S. Pat.: www.alconpatents.com
© 2023 Alcon Inc.
Alcon
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
EYSUVIS [eye-SU-vis]
(loteprednol etabonate ophthalmic suspension) 0.25%
for topical ophthalmic useThis Instructions for Use contains information on how to properly administer EYSUVIS.
Important Information You Need to Know Before Using EYSUVIS
- EYSUVIS is for use in the eye.
- Wash your hands before using EYSUVIS.
- Do not use if the tamper-evident seal is not intact.
- Do not let the EYSUVIS dropper tip touch your eye, fingers, or any other surfaces to avoid contamination or injury to your eye.
- Use EYSUVIS exactly as your doctor tells you to.
- If you are using EYSUVIS with other eye (ophthalmic) medicines, you should wait at least 5 minutes between using EYSUVIS and the other medicine.
- If you wear contact lenses, remove them before using EYSUVIS.
- Put the pink cap back on EYSUVIS after each use.
Before you use EYSUVIS for the first time:
There are two caps on your bottle of EYSUVIS. Hold the bottle firmly by its neck. Remove the white cap by twisting it clockwise (See Figure A). Throw away the white cap. EYSUVIS is now ready to use.
Follow Steps 1 to 6 each time you use EYSUVIS.
- Wash your hands well.
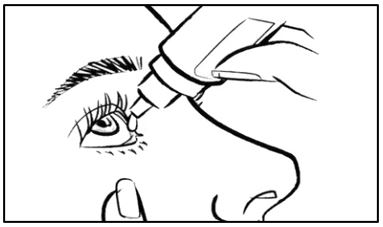
- Shake the EYSUVIS bottle for 2 to 3 seconds before using (See Figure B).
- Remove the pink cap from the top of the EYSUVIS dropper by turning it counterclockwise (See Figure C). Keep the pink cap. Do not let the EYSUVIS dropper tip touch your eye, fingers, or any other surface.
- Turn the EYSUVIS bottle upside down (See Figure D).
- Tilt your head back. Hold the bottle directly above your affected eye. Squeeze the middle of the EYSUVIS bottle gently to put 1 to 2 drops (follow your doctor's instruction) into the affected eye (See Figure E).
- Place the pink cap back onto the EYSUVIS bottle and tighten by turning clockwise (See Figure F).
If you use contact lenses, wait for 15 minutes before placing them back in.
How should I store EYSUVIS?
- Store EYSUVIS upright between 59ºF to 77ºF (15ºC to 25ºC).
- Do not freeze.
- After opening, EYSUVIS can be used until the expiration date (EXP) on the bottle. The expiration date can be found on the lower right side of the label on the bottle.
Keep EYSUVIS and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
Approved 11/2023
-
PRINCIPAL DISPLAY PANEL - NDC: 71571-333-83 - Bottle Label
NDC: 71571-333-83 Rx Only
EYsuVIS®
(loteprednol etabonate
ophthalmic suspension) 0.25%
Sterile
8.3 mL
FOR TOPICAL APPLICATION
IN THE EYE.
Dosage: See Prescribing Information.
Manufactured for: Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA

-
PRINCIPAL DISPLAY PANEL - NDC: 71571-333-83 - Carton Label
NDC: 71571-333-83 Rx Only
EYsuVIS®
(loteprednol etabonate
ophthalmic suspension) 0.25%
FOR TOPICAL APPLICATION
IN THE EYE.
Sterile
8.3 mL
Alcon
Dosage: See Prescribing Information.
SHAKE FOR 2 TO 3 SECONDS
Storage: Store upright at
15°C to 25°C (59°F to 77°F).
Do not freeze.
DO NOT USE IF
TAMPER-EVIDENT
OVERCAP IS NOT INTACT
300064649-1123
Store Upright
Manufactured for:
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
- PRINCIPAL DISPLAY PANEL - NDC: 71571-333-24 - Bottle Label
- PRINCIPAL DISPLAY PANEL - NDC: 71571-333-24 - Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 71571-333-01 - Bottle Label
- PRINCIPAL DISPLAY PANEL - NDC: 71571-333-01 - Carton Label
-
INGREDIENTS AND APPEARANCE
EYSUVIS
loteprednol etabonate suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71571-333 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOTEPREDNOL ETABONATE (UNII: YEH1EZ96K6) (LOTEPREDNOL - UNII:Z8CBU6KR16) LOTEPREDNOL ETABONATE 2.5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71571-333-83 1 in 1 CARTON 11/18/2020 1 8.3 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 71571-333-24 2.4 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 11/18/2020 3 NDC: 71571-333-01 1 in 1 CARTON 12/14/2023 3 1 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210933 11/18/2020 Labeler - ALCON LABORATORIES, INC. (008018525)
Trademark Results [Eysuvis]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EYSUVIS 90126591 not registered Live/Pending |
Kala Pharmaceuticals, Inc. 2020-08-20 |
 EYSUVIS 90126587 not registered Live/Pending |
Kala Pharmaceuticals, Inc. 2020-08-20 |
 EYSUVIS 88708748 not registered Live/Pending |
Kala Pharmaceuticals, Inc. 2019-11-27 |
 EYSUVIS 88708726 not registered Live/Pending |
Kala Pharmaceuticals, Inc. 2019-11-27 |
 EYSUVIS 87694659 not registered Live/Pending |
Kala Pharmaceuticals, Inc. 2017-11-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.