SUPRAX- cefixime capsule
SUPRAX by
Drug Labeling and Warnings
SUPRAX by is a Prescription medication manufactured, distributed, or labeled by REMEDYREPACK INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SUPRAX® safely and effectively. See full prescribing information for SUPRAX®.
SUPRAX® (cefixime) tablets, for oral use
SUPRAX® (cefixime) capsules, for oral use
SUPRAX® (cefixime) chewable tablets, for oral use
SUPRAX® (cefixime) for oral suspension
Initial U.S. Approval: 1986RECENT MAJOR CHANGES
Warnings and Precautions, Risk in Patients with Phenylketonuria ( 5.6) 03/2017
INDICATIONS AND USAGE
SUPRAX (cefixime) is a cephalosporin antibacterial drug indicated in the treatment of adults and pediatric patients six months and older with the following infections:
- Uncomplicated Urinary Tract Infections ( 1.1)
- Otitis Media ( 1.2)
- Pharyngitis and Tonsillitis ( 1.3)
- Acute Exacerbations of Chronic Bronchitis ( 1.4)
- Uncomplicated Gonorrhea (cervical/urethral) ( 1.5)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of SUPRAX and other antibacterial drugs, SUPRAX should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. ( 1.6)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Contraindicated in patients with known allergy to cefixime or other cephalosporins. ( 4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions including shock and fatalities have been reported with cefixime. Discontinue use if a reaction occurs. ( 5.1)
- Clostridium difficile associated diarrhea: Evaluate if diarrhea occurs. ( 5.2)
- Risk in Patients with Phenylketonuria (PKU): Before prescribing SUPRAX chewable tablets in a patient with PKU, consider the combined daily amount of phenylalanine from all sources, including SUPRAX chewable tablets. ( 5.6)
ADVERSE REACTIONS
Most common adverse reactions are gastrointestinal such as diarrhea (16%), nausea (7%), loose stools (6%), abdominal pain (3%), dyspepsia (3%), and vomiting. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharma at 1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy: Cefixime should be used during pregnancy only if clearly needed. ( 8.1)
- Nursing Mothers: Consideration should be given to discontinuing nursing temporarily during treatment with cefixime. ( 8.3)
- Children: Efficacy and safety in infants aged less than six months have not been established. ( 8.4)
- Geriatric Use: Clinical studies did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. ( 8.5)
- Renal Impairment: Cefixime may be administered in the presence of impaired renal function. Dose adjustment is required in patients whose creatinine clearance is less than 60 mL/min. ( 8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Uncomplicated Urinary Tract Infections
1.2 Otitis Media
1.3 Pharyngitis and Tonsillitis
1.4 Acute Exacerbations of Chronic Bronchitis
1.5 Uncomplicated Gonorrhea (cervical/urethral)
1.6 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Adults
2.2 Pediatric Patients (6 months or older)
2.3 Renal Impairment
2.4 Reconstitution Directions for Oral Suspension
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Clostridium difficile-Associated Diarrhea
5.3 Dose Adjustment in Renal Impairment
5.4 Coagulation Effects
5.5 Development of Drug-Resistant Bacteria
5.6 Risk in Patients with Phenylketonuria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Carbamazepine
7.2 Warfarin and Anticoagulants
7.3 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Uncomplicated Urinary Tract Infections
SUPRAX is indicated in the treatment of adults and pediatric patients six months of age or older with uncomplicated urinary tract infections caused by susceptible isolates of Escherichia coli and Proteus mirabilis.
1.2 Otitis Media
SUPRAX is indicated in the treatment of adults and pediatric patients six months of age or older with otitis media caused by susceptible isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pyogenes. (Efficacy for Streptococcus pyogenes in this organ system was studied in fewer than 10 infections.)
Note: For patients with otitis media caused by Streptococcus pneumoniae, overall response was approximately 10% lower for cefixime than for the comparator [see Clinical Studies ( 14)].
1.3 Pharyngitis and Tonsillitis
SUPRAX is indicated in the treatment of adults and pediatric patients six months of age or older with pharyngitis and tonsillitis caused by susceptible isolates of Streptococcus pyogenes. (Note: Penicillin is the usual drug of choice in the treatment of Streptococcus pyogenes infections. SUPRAX is generally effective in the eradication of Streptococcus pyogenes from the nasopharynx; however, data establishing the efficacy of SUPRAX in the subsequent prevention of rheumatic fever is not available.)
1.4 Acute Exacerbations of Chronic Bronchitis
SUPRAX is indicated in the treatment of adults and pediatric patients six months of age or older with acute exacerbations of chronic bronchitis caused by susceptible isolates of Streptococcus pneumoniae and Haemophilus influenzae.
1.5 Uncomplicated Gonorrhea (cervical/urethral)
SUPRAX is indicated in the treatment of adults and pediatric patients six months of age or older with uncomplicated gonorrhea (cervical/urethral) caused by susceptible isolates of Neisseria gonorrhoeae (penicillinase-and non-penicillinase-producing isolates).
1.6 Usage
To reduce the development of drug resistant bacteria and maintain the effectiveness of SUPRAX and other antibacterial drugs, SUPRAX should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antimicrobial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Adults
The recommended dose of cefixime is 400 mg daily. This may be given as a 400 mg tablet or capsule daily or the 400 mg tablet may be split and given as one half tablet every 12 hours. For the treatment of uncomplicated cervical/urethral gonococcal infections, a single oral dose of 400 mg is recommended. The capsule and tablet may be administered without regard to food.
In the treatment of infections due to Streptococcus pyogenes, a therapeutic dosage of cefixime should be administered for at least 10 days.
2.2 Pediatric Patients (6 months or older)
The recommended dose is 8 mg/kg/day of the suspension. This may be administered as a single daily dose or may be given in two divided doses, as 4 mg/kg every 12 hours.
Note: A suggested dose has been determined for each pediatric weight range. Refer to Table 1. Ensure all orders that specify a dose in milliliters include a concentration, because SUPRAX for oral suspension is available in three different concentrations (100 mg/5 mL, 200 mg/5 mL, and 500 mg/5 mL).
Table 1. Suggested doses for pediatric patients - * The preferred concentrations of oral suspension to use are 100 mg/5 mL or 200 mg/5 mL for pediatric patients in these weight ranges.
PEDIATRIC DOSAGE CHART
Doses are suggested for each weight range and rounded for ease of administration
SUPRAX (cefixime) for oral suspension
SUPRAX (cefixime) chewable tablet
100 mg/5 mL
200 mg/5 mL
500 mg/5 mL
Patient Weight
(kg)
Dose/Day
(mg)
Dose/Day
(mL)
Dose/Day
(mL)
Dose/Day
(mL)
Dose
5 to 7.5 *
50
2.5
--
--
--
7.6 to 10 *
80
4
2
--
--
10.1 to 12.5
100
5
2.5
1
1 tablet of 100 mg
12.6 to 20.5
150
7.5
4
1.5
1 tablet of 150 mg
20.6 to 28
200
10
5
2
1 tablet of 200 mg
28.1 to 33
250
12.5
6
2.5
1 tablet of 100 mg and
1 tablet of 150 mg
33.1 to 40
300
15
7.5
3
2 tablets of 150 mg
40.1 to 45
350
17.5
9
3.5
1 tablet of 150 mg and
1 tablet of 200 mg
45.1 or greater
400
20
10
4
2 tablets of 200 mg
Children weighing more than 45 kg or older than 12 years should be treated with the recommended adult dose. SUPRAX (cefixime) chewable tablets must be chewed or crushed before swallowing.
Otitis media should be treated with the chewable tablets or suspension. Clinical trials of otitis media were conducted with the chewable tablets or suspension, and the chewable tablets or suspension results in higher peak blood levels than the tablet when administered at the same dose.
Therefore, the tablet or capsule should not be substituted for the chewable tablets or suspension in the treatment of otitis media [see Clinical Pharmacology ( 12.3)] .
In the treatment of infections due to Streptococcus pyogenes, a therapeutic dosage of cefixime should be administered for at least 10 days.
2.3 Renal Impairment
SUPRAX may be administered in the presence of impaired renal function. Normal dose and schedule may be employed in patients with creatinine clearances of 60 mL/min or greater. Refer to Table 2 for dose adjustments for adults with renal impairment. Neither hemodialysis nor peritoneal dialysis removes significant amounts of drug from the body.
Table 2. Doses for Adults with Renal Impairment - * The preferred concentrations of oral suspension to use are 200 mg/5 mL or 500 mg/5 mL for patients with this renal dysfunction
Renal Dysfunction
SUPRAX (cefixime) for oral suspension
Tablet
Chewable Tablet
Creatinine Clearance (mL/min)
100 mg/5 mL
200 mg/5 mL
500 mg/5 mL
400 mg
200 mg
Dose/Day (mL)
Dose/Day (mL)
Dose/Day (mL)
Dose/Day
Dose/Day
60 or greater
Normal dose
Normal dose
Normal dose
Normal dose
Normal dose
21 to 59 *
OR renal hemodialysis *
13
6.5
2.6
Not Appropriate
Not Appropriate
20 or less
OR continuous peritoneal dialysis
8.6
4.4
1.8
0.5 tablet
1 tablet
2.4 Reconstitution Directions for Oral Suspension
Strength
Bottle Size
Reconstitution Directions
100 mg/5 mL and
200 mg/5 mL
100 mL
To reconstitute, suspend with 68 mL water.
Method: Tap the bottle several times to loosen powder contents prior to reconstitution.
Add approximately half the total amount of water for reconstitution and shake well.
Add the remainder of water and shake well.
100 mg/5 mL and
200 mg/5 mL
75 mL
To reconstitute, suspend with 51 mL water.
Method: Tap the bottle several times to loosen powder contents prior to reconstitution.
Add approximately half the total amount of water for reconstitution and shake well.
Add the remainder of water and shake well.
100 mg/5 mL and
200 mg/5 mL
50 mL
To reconstitute, suspend with 34 mL water.
Method: Tap the bottle several times to loosen powder contents prior to reconstitution.
Add approximately half the total amount of water for reconstitution and shake well.
Add the remainder of water and shake well.
200 mg/5 mL
37.5 mL
To reconstitute, suspend with 26 mL water.
Method: Tap the bottle several times to loosen powder contents prior to reconstitution.
Add approximately half the total amount of water for reconstitution and shake well.
Add the remainder of water and shake well.
200 mg/5 mL
25 mL
To reconstitute, suspend with 17 mL water.
Method: Tap the bottle several times to loosen powder contents prior to reconstitution.
Add approximately half the total amount of water for reconstitution and shake well.
Add the remainder of water and shake well.
500 mg/5 mL
20 mL
To reconstitute, suspend with 14 mL water.
Method: Tap the bottle several times to loosen powder contents prior to reconstitution.
Add approximately half the total amount of water for reconstitution and shake well.
Add the remainder of water and shake well.
500 mg/5 mL
10 mL
To reconstitute, suspend with 8 mL water.
Method: Tap the bottle several times to loosen powder contents prior to reconstitution.
Add approximately half the total amount of water for reconstitution and shake well.
Add the remainder of water and shake well.
After reconstitution, the suspension may be kept for 14 days either at room temperature, or under refrigeration, without significant loss of potency. Keep tightly closed. Shake well before using. Discard unused portion after 14 days.
-
3 DOSAGE FORMS AND STRENGTHS
SUPRAX is available for oral administration in the following dosage forms and strengths:
- Film-coated tablets provide 400 mg of cefixime as trihydrate. These are white to off-white, film-coated, capsule shaped tablets with beveled edges and a divided score line on each side. The tablet is debossed with "SUPRAX" across one side and "LUPIN" across the other side.
- Capsules provide 400 mg of cefixime as trihydrate. These are size "00EL" capsules with pink opaque cap and pink opaque body with "LU" on the cap and "U43" on the body in black ink. Capsules contain white to yellowish white granular powder.
- Chewable tablets provide either 100 mg or 150 mg or 200 mg of cefixime as trihydrate. The 100 mg tablet is pink, round tablet, debossed with "SUPRAX 100" on one side and "LUPIN" on other side. The 150 mg tablet is pink, round tablet, debossed with "SUPRAX 150" on one side and "LUPIN" on other side. The 200 mg tablet is pink, round tablet, debossed with "SUPRAX 200" on one side and "LUPIN" on other side.
- Powder for oral suspension, when reconstituted, provides either 100 mg/5 mL or 200 mg/5 mL or 500 mg/5 mL of cefixime as trihydrate. For 100 mg/5 mL and 200 mg/5 mL, the powder has an off white to pale yellow color and is strawberry flavored. For 500 mg/5 mL, the powder has an off white to cream color and is strawberry flavored.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Anaphylactic/anaphylactoid reactions (including shock and fatalities) have been reported with the use of cefixime.
Before therapy with SUPRAX is instituted, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to cephalosporins, penicillins, or other drugs. If this product is to be given to penicillin-sensitive patients, caution should be exercised because cross hypersensitivity among beta-lactam antibacterial drugs has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to SUPRAX occurs, discontinue the drug.
5.2 Clostridium difficile-Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including SUPRAX, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing isolates of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Dose Adjustment in Renal Impairment
The dose of SUPRAX should be adjusted in patients with renal impairment as well as those undergoing continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis (HD). Patients on dialysis should be monitored carefully [see Dosage and Administration ( 2)] .
5.4 Coagulation Effects
Cephalosporins, including SUPRAX, may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
5.5 Development of Drug-Resistant Bacteria
Prescribing Suprax (cefixime) in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.6 Risk in Patients with Phenylketonuria
Phenylalanine can be harmful to patients with phenylketonuria (PKU). SUPRAX chewable tablets contain aspartame, a source of phenylalanine. Each 100 mg, 150 mg and 200 mg strength contains 3.3 mg, 5 mg and 6.7 mg of phenylalanine, respectively. Before prescribing SUPRAX chewable tablets in a patient with PKU, consider the combined daily amount of phenylalanine from all sources, including SUPRAX chewable tablets.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most commonly seen adverse reactions in U.S. trials of the tablet formulation were gastrointestinal events, which were reported in 30% of adult patients on either the twice daily or the once daily regimen. Five percent (5%) of patients in the U.S. clinical trials discontinued therapy because of drug-related adverse reactions. Individual adverse reactions included diarrhea 16%, loose or frequent stools 6%, abdominal pain 3%, nausea 7%, dyspepsia 3%, and flatulence 4%. The incidence of gastrointestinal adverse reactions, including diarrhea and loose stools, in pediatric patients receiving the suspension was comparable to the incidence seen in adult patients receiving tablets.
6.2 Post-marketing Experience
The following adverse reactions have been reported following the post-approval use of cefixime. Incidence rates were less than 1 in 50 (less than 2%).
Gastrointestinal
Several cases of documented pseudomembranous colitis were identified in clinical trials. The onset of pseudomembranous colitis symptoms may occur during or after therapy.
Hypersensitivity Reactions
Anaphylactic/anaphylactoid reactions (including shock and fatalities), skin rashes, urticaria, drug fever, pruritus, angioedema, and facial edema. Erythema multiforme, Stevens-Johnson syndrome, and serum sickness-like reactions have been reported.
Hepatic
Transient elevations in SGPT, SGOT, alkaline phosphatase, hepatitis, jaundice.
Renal
Transient elevations in BUN or creatinine, acute renal failure.
Central Nervous System
Headaches, dizziness, seizures.
Hemic and Lymphatic System
Transient thrombocytopenia, leukopenia, neutropenia, prolongation in prothrombin time, elevated LDH, pancytopenia, agranulocytosis, and eosinophilia.
Abnormal Laboratory Tests
Hyperbilirubinemia.
Other Adverse Reactions
Genital pruritus, vaginitis, candidiasis, toxic epidermal necrolysis.
Adverse Reactions Reported for Cephalosporin-class Drugs
Allergic reactions, superinfection, renal dysfunction, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemolytic anemia, hemorrhage, and colitis.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced [see Dosage and Administration ( 2) and Overdosage ( 10)] . If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
-
7 DRUG INTERACTIONS
7.1 Carbamazepine
Elevated carbamazepine levels have been reported in postmarketing experience when cefixime is administered concomitantly. Drug monitoring may be of assistance in detecting alterations in carbamazepine plasma concentrations.
7.2 Warfarin and Anticoagulants
Increased prothrombin time, with or without clinical bleeding, has been reported when cefixime is administered concomitantly.
7.3 Drug/Laboratory Test Interactions
A false-positive reaction for ketones in the urine may occur with tests using nitroprusside but not with those using nitroferricyanide.
The administration of cefixime may result in a false-positive reaction for glucose in the urine using Clinitest ®**, Benedict's solution, or Fehling's solution. It is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as Clinistix ®** or TesTape ®**) be used. A false-positive direct Coombs test has been reported during treatment with other cephalosporins; therefore, it should be recognized that a positive Coombs test may be due to the drug.
**Clinitest ® and Clinistix ® are registered trademarks of Ames Division, Miles Laboratories, Inc. Tes-Tape ® is a registered trademark of Eli Lilly and Company.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Reproduction studies have been performed in mice and rats at doses up to 40 times the human dose and have revealed no evidence of harm to the fetus due to cefixime. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.2 Labor and Delivery
Cefixime has not been studied for use during labor and delivery. Treatment should only be given if clearly needed.
8.3 Nursing Mothers
It is not known whether cefixime is excreted in human milk. Consideration should be given to discontinuing nursing temporarily during treatment with this drug.
8.4 Pediatric Use
Safety and effectiveness of cefixime in children aged less than six months old have not been established. The incidence of gastrointestinal adverse reactions, including diarrhea and loose stools, in the pediatric patients receiving the suspension, was comparable to the incidence seen in adult patients receiving tablets.
8.5 Geriatric Use
Clinical studies did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. A pharmacokinetic study in the elderly detected differences in pharmacokinetic parameters [see Clinical Pharmacology ( 12.3)] . These differences were small and do not indicate a need for dosage adjustment of the drug in the elderly.
8.6 Renal Impairment
The dose of cefixime should be adjusted in patients with renal impairment as well as those undergoing continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis (HD). Patients on dialysis should be monitored carefully [see Dosage and Administration ( 2.3)] .
-
10 OVERDOSAGE
Gastric lavage may be indicated; otherwise, no specific antidote exists. Cefixime is not removed in significant quantities from the circulation by hemodialysis or peritoneal dialysis. Adverse reactions in small numbers of healthy adult volunteers receiving single doses up to 2 g of cefixime did not differ from the profile seen in patients treated at the recommended doses.
-
11 DESCRIPTION
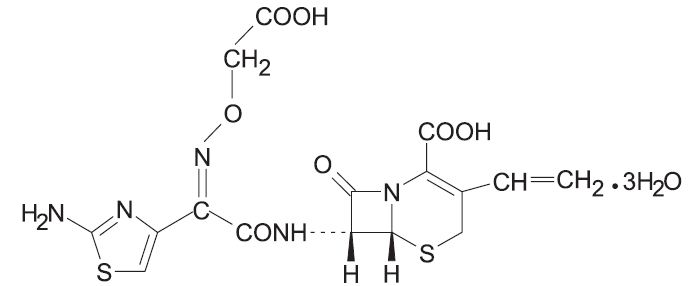
Cefixime is a semisynthetic, cephalosporin antibacterial for oral administration. Chemically, it is ( 6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-8-oxo-3-vinyl-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid, 7 2-( Z)-[ O-(carboxy methyl) oxime] trihydrate.
Molecular weight = 507.50 as the trihydrate. Chemical Formula is C 16H 15N 5O 7S 2.3H 2O
The structural formula for cefixime is:
- Inactive ingredients contained in SUPRAX ® (cefixime) 400 mg tablets USP are: dibasic calcium phosphate, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch, titanium dioxide, and triacetin.
- Inactive ingredients contained in SUPRAX ® (cefixime) 400 mg capsules are: colloidal silicon dioxide, crospovidone, low substituted hydroxy propyl cellulose, magnesium stearate, and mannitol. The capsule shell contains the following inactive ingredients: ferric oxide black, ferric oxide red, gelatin, potassium hydroxide, propylene glycol, shellac, sodium lauryl sulfate, and titanium dioxide.
- Inactive ingredients contained in SUPRAX ® (cefixime) 100 mg or 150 mg or 200 mg chewable tablets are: aspartame, colloidal silicon dioxide, crospovidone, FD&C Red # 40 Aluminium Lake, low substituted hydroxypropyl cellulose, magnesium stearate, mannitol, fantasy flavor permaseal, and tutti frutti flavor.
- Inactive ingredients contained in SUPRAX ® (cefixime) powder for oral suspension USP are: colloidal silicon dioxide, sodium benzoate, strawberry flavor, sucralose (only in 500 mg/5 mL strength), sucrose, and xanthan gum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cefixime is a semisynthetic cephalosporin antibacterial drug [see Microbiology ( 12.4)] .
12.3 Pharmacokinetics
SUPRAX chewable tablets are bioequivalent to oral suspension.
SUPRAX tablets and suspension, given orally, are about 40% to 50% absorbed whether administered with or without food; however, time to maximal absorption is increased approximately 0.8 hours when administered with food. A single 200 mg tablet of cefixime produces an average peak serum concentration of approximately 2 mcg/mL (range 1 to 4 mcg/mL); a single 400 mg tablet produces an average peak concentration of approximately 3.7 mcg/mL (range 1.3 to 7.7 mcg/mL). The oral suspension produces average peak concentrations approximately 25% to 50% higher than the tablets, when tested in normal adult volunteers. Two hundred and 400 mg doses of oral suspension produce average peak concentrations of 3 mcg/mL (range 1 to 4.5 mcg/mL) and 4.6 mcg/mL (range 1.9 to 7.7 mcg/mL), respectively, when tested in normal adult volunteers. The area under the time versus concentration curve (AUC) is greater by approximately 10% to 25% with the oral suspension than with the tablet after doses of 100 to 400 mg, when tested in normal adult volunteers. This increased absorption should be taken into consideration if the oral suspension is to be substituted for the tablet. Because of the lack of bioequivalence, tablets should not be substituted for oral suspension in the treatment of otitis media [see Dosage and Administration ( 2)] . Cross-over studies of tablet versus suspension have not been performed in children.
The 400 mg capsule is bioequivalent to the 400 mg tablet under fasting conditions. However, food reduces the absorption following administration of the capsule by approximately 15% based on AUC and 25% based on C max.
Peak serum concentrations occur between 2 and 6 hours following oral administration of a single 200 mg tablet, a single 400 mg tablet or 400 mg of cefixime suspension. Peak serum concentrations occur between 2 and 5 hours following a single administration of 200 mg of suspension. Peak serum concentrations occur between 3 and 8 hours following oral administration of a single 400 mg capsule.
Distribution
Serum protein binding is concentration independent with a bound fraction of approximately 65%. In a multiple dose study conducted with a research formulation which is less bioavailable than the tablet or suspension, there was little accumulation of drug in serum or urine after dosing for 14 days. Adequate data on CSF levels of cefixime are not available.
Metabolism and Excretion
There is no evidence of metabolism of cefixime in vivo. Approximately 50% of the absorbed dose is excreted unchanged in the urine in 24 hours. In animal studies, it was noted that cefixime is also excreted in the bile in excess of 10% of the administered dose. The serum half-life of cefixime in healthy subjects is independent of dosage form and averages 3 to 4 hours but may range up to 9 hours in some normal volunteers.
Special Populations
Geriatrics: Average AUCs at steady state in elderly patients are approximately 40% higher than average AUCs in other healthy adults. Differences in the pharmacokinetic parameters between 12 young and 12 elderly subjects who received 400 mg of cefixime once daily for 5 days are summarized as follows:
- * Difference between age groups was significant. (p<0.05)
Pharmacokinetic Parameters (mean ± SD) for Cefixime in Both Young & Elderly Subjects
Pharmacokinetic parameter
Young
Elderly
C max (mg/L)
4.74 ± 1.43
5.68 ± 1.83
T max (h) *
3.9 ± 0.3
4.3 ± 0.6
AUC (mg.h/L) *
34.9 ± 12.2
49.5 ± 19.1
T ½ (h) *
3.5 ± 0.6
4.2 ± 0.4
C ave (mg/L) *
1.42 ±0.50
1.99 ± 0.75
However, these increases were not clinically significant [see Dosage and Administration ( 2)] .
Renal Impairment: In subjects with moderate impairment of renal function (20 to 40 mL/min creatinine clearance), the average serum half-life of cefixime is prolonged to 6.4 hours. In severe renal impairment (5 to 20 mL/min creatinine clearance), the half-life increased to an average of 11.5 hours. The drug is not cleared significantly from the blood by hemodialysis or peritoneal dialysis. However, a study indicated that with doses of 400 mg, patients undergoing hemodialysis have similar blood profiles as subjects with creatinine clearances of 21 to 60 mL/min.
12.4 Microbiology
As with other cephalosporins, the bactericidal action of cefixime results from inhibition of cell wall synthesis. Cefixime is stable in the presence of certain beta-lactamase enzymes. As a result, certain organisms resistant to penicillins and some cephalosporins due to the presence of beta-lactamases may be susceptible to cefixime.
Resistance
Resistance to cefixime in isolates of Haemophilus influenzae and Neisseria gonorrhoeae is most often associated with alterations in penicillin-binding proteins (PBPs). Cefixime may have limited activity against Enterobacteriaceae producing extended spectrum beta-lactamases (ESBLs). Pseudomonas species, Enterococcus species, strains of Group D streptococci, Listeria monocytogenes, most strains of staphylococci (including methicillin-resistant strains), most strains of Enterobacter species, most strains of Bacteroides fragilis, and most strains of Clostridium species are resistant to cefixime.
Antimicrobial Activity
Cefixime has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage ( 1)].
Gram-positive Bacteria
Streptococcus pneumoniae
Streptococcus pyogenes
Gram-negative Bacteria
Escherichia coli
Haemophilus influenzae
Moraxella catarrhalis
Neisseria gonorrhoeae
Proteus mirabilis
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for cefixime against isolates of similar genus or organism group. However, the efficacy of cefixime in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Gram-positive Bacteria
Streptococcus agalactiae
Gram-negative Bacteria
Citrobacter amalonaticus
Citrobacter diversus
Haemophilus parainfluenzae
Klebsiella oxytoca
Klebsiella pneumoniae
Pasteurella multocida
Proteus vulgaris
Providencia species
Salmonella species
Serratia marcescens
Shigella species
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime studies in animals to evaluate carcinogenic potential have not been conducted. Cefixime did not cause point mutations in bacteria or mammalian cells, DNA damage, or chromosome damage in vitro and did not exhibit clastogenic potential in vivo in the mouse micronucleus test. In rats, fertility and reproductive performance were not affected by cefixime at doses up to 25 times the adult therapeutic dose.
-
14 CLINICAL STUDIES
Comparative clinical trials of otitis media were conducted in nearly 400 children between the ages of 6 months to 10 years. Streptococcus pneumoniae was isolated from 47% of the patients, Haemophilus influenzae from 34%, Moraxella catarrhalis from 15% and S. pyogenes from 4%.
The overall response rate of Streptococcus pneumoniae to cefixime was approximately 10% lower and that of Haemophilus influenzae or Moraxella catarrhalis approximately 7% higher (12% when beta-lactamase positive isolates of H. influenzae are included) than the response rates of these organisms to the active control drugs.
In these studies, patients were randomized and treated with either cefixime at dose regimens of 4 mg/kg twice a day or 8 mg/kg once a day, or with a comparator. Sixty-nine to 70% of the patients in each group had resolution of signs and symptoms of otitis media when evaluated 2 to 4 weeks post-treatment, but persistent effusion was found in 15% of the patients. When evaluated at the completion of therapy, 17% of patients receiving cefixime and 14% of patients receiving effective comparative drugs (18% including those patients who had Haemophilus influenzae resistant to the control drug and who received the control antibacterial drug) were considered to be treatment failures. By the 2 to 4 week follow-up, a total of 30%-31% of patients had evidence of either treatment failure or recurrent disease.
(a)Number eradicated/number isolated.
(b)An additional 20 beta-lactamase positive isolates of Haemophilus influenzae were isolated, but were excluded from this analysis because they were resistant to the control antibacterial drug. In nineteen of these, the clinical course could be assessed and a favorable outcome occurred in 10. When these cases are included in the overall bacteriological evaluation of therapy with the control drugs, 140/185 (76%) of pathogens were considered to be eradicated.
Bacteriological Outcome of Otitis Media at Two to Four Weeks Post-Therapy Based on Repeat Middle Ear Fluid Culture or Extrapolation from Clinical Outcome
Organism
Cefixime(a)
4 mg/kg BID
Cefixime(a)
8 mg/kg QD
Control(a)
drugs
Streptococcus pneumoniae
48/70 (69%)
18/22 (82%)
82/100 (82%)
Haemophilus influenzae
beta-lactamase negative
24/34 (71%)
13/17 (76%)
23/34 (68%)
Haemophilus influenzae
beta-lactamase positive
17/22 (77%)
9/12 (75%)
1/1 (b)
Moraxella catarrhalis
26/31 (84%)
5/5
18/24 (75%)
S. pyogenes
5/5
3/3
6/7
All Isolates
120/162 (74%)
48/59 (81%)
130/166 (78%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
SUPRAX ® is available for oral administration in following dosage forms, strengths and packages listed in the table below:
Dosage Form
Strength
Description
Package Size
NDC Code
Storage
White to off-white,
film-coated, capsule
shaped tablets with
beveled edges and
Bottles of
10 tablets
27437-201-10
SUPRAX® (cefixime)
tablets USP
400 mg
a divided score
line on each side,
debossed with “SUPRAX”
across one side
Bottle of
50 tablets
27437-201-08
Store at 20 to 25°C
(68 to 77°F)
[See USP Controlled
Room Temperature].
and “LUPIN” across
other side, containing
400 mg of cefixime
as the trihydrate.
Bottle of
100 tablets
27437-201-01
SUPRAX® (cefixime)
capsules
400 mg
Size “00EL” capsules with
pink opaque cap and pink
opaque body, imprinted
with “LU” on cap and
“U43” on body in black
Bottle of
50 capsules
27437-208-08
Store at 20 to 25°C
(68 to 77°F)
[See USP Controlled
Room Temperature].
ink, containing white to
yellowish white granular
powder containing
400 mg of cefixime as the
trihydrate.
Unit Dose
Package of 10
(1 blister of
10 capsules)
27437-208-11
Bottles of
10 tablets
27437-203-10
100 mg
Pink, round tablet,
debossed with “SUPRAX 100”
on one side and “LUPIN”
on other side.
Bottle of
50 tablets
27437-203-08
Unit Dose
Package of 10
(1 blister of
10 tablets)
27437-203-11
Bottles of
10 tablets
27437-204-10
SUPRAX® (cefixime)
chewable tablets
150 mg
Pink, round tablet,
debossed with “SUPRAX 150”
on one side and “LUPIN”
on other side.
Bottle of
50 tablets
27437-204-08
Store at 20 to 25°C
(68 to 77°F)
[See USP Controlled
Room Temperature].
Unit Dose
Package of 10
(1 blister of
10 tablets)
27437-204-11
Bottles of
10 tablets
27437-205-10
200 mg
Pink, round tablet,
debossed with “SUPRAX 200”
on one side and “LUPIN”
on other side.
Bottle of
50 tablets
27437-205-08
Unit Dose
Package of 10
(1 blister of
10 tablets)
27437-205-11
Off-white to pale
yellow colored powder.
After reconstituted as
Bottle of
50 mL
68180-202-03
100 mg/5 mL
directed, each 5 mL of
reconstituted suspension
contains 100 mg of
Bottle of
75 mL
68180-202-02
cefixime as the trihydrate.
Bottle of
100 mL
68180-202-01
Bottle of
25 mL
27437-206-05
Bottle of
37.5 mL
27437-206-06
Prior to reconstitution:
Store drug powder at
SUPRAX® (cefixime)
for oral
suspension USP
200 mg/5 mL
Off-white to pale
yellow colored powder.
After reconstituted as
directed, each 5 mL of
Bottle of
50 mL
27437-206-03
20 to 25°C (68 to 77°F)
[See USP Controlled
Room Temperature].
reconstituted suspension
contains 200 mg of
cefixime as the trihydrate.
Bottle of
75 mL
27437-206-02
After reconstitution:
Store at room temperature
or under refrigeration.
Keep tightly closed.
Bottle of
100 mL
27437-206-01
500 mg/5 mL
Off white to cream
colored powder forming
off-white to pale yellow
suspension with
characteristic fruity odor
Bottle of
10 mL
27437-207-02
on constitution. After
reconstituted as directed,
each mL of reconstituted
suspension contains
100 mg of cefixime
as the trihydrate.
Bottle of
20 mL
27437-207-03
-
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
Counsel patients that antibacterial drugs, including cefixime, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefixime is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefixime for oral suspension or cefixime chewable tablets or other antibacterial drugs in the future.
Counsel patients with phenylketonuria that SUPRAX chewable tablets contain aspartame, a source of phenylalanine as follows: Each SUPRAX chewable tablet contains 3.3 mg, 5 mg and 6.7 mg of phenylalanine per 100 mg, 150 mg and 200 mg strength, respectively.
Advise patients that diarrhea is a common problem caused by antibacterial drugs which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
-
SPL UNCLASSIFIED SECTION
Products
Manufactured for:
Manufactured by:
SUPRAX ® (cefixime) tablets, 400 mg
SUPRAX ® (cefixime) capsules, 400 mg
Lupin Pharma
SUPRAX ® (cefixime) chewable tablets, 100 mg, 150 mg and 200 mg
Baltimore, Maryland 21202
Lupin Limited
SUPRAX ® (cefixime) for oral suspension, 200 mg/5 mL
United States.
Mandideep 462 046
SUPRAX ® (cefixime) for oral suspension, 500 mg/5 mL
India.
SUPRAX ® (cefixime) for oral suspension, 100 mg/5 mL
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States.
-
PRINCIPAL DISPLAY PANEL
DRUG: SUPRAX
GENERIC: cefixime
DOSAGE: CAPSULE
ADMINSTRATION: ORAL
NDC: 70518-2006-0
COLOR: pink
SHAPE: CAPSULE
SCORE: No score
SIZE: 26 mm
IMPRINT: LU;U43
PACKAGING: 1 in 1 BOTTLE, PLASTIC
ACTIVE INGREDIENT(S):
- CEFIXIME 400mg in 1
INACTIVE INGREDIENT(S):
- CROSPOVIDONE
- PROPYLENE GLYCOL
- SHELLAC
- POTASSIUM HYDROXIDE
- SILICON DIOXIDE
- SODIUM LAURYL SULFATE
- MANNITOL
- FERRIC OXIDE RED
- FERROSOFERRIC OXIDE
- GELATIN
- HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED
- MAGNESIUM STEARATE
- TITANIUM DIOXIDE

-
INGREDIENTS AND APPEARANCE
SUPRAX
cefixime capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70518-2006(NDC:27437-208) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFIXIME (UNII: 97I1C92E55) (CEFIXIME ANHYDROUS - UNII:XZ7BG04GJX) CEFIXIME ANHYDROUS 400 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 2S7830E561) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink (Pink Opaque Cap) , pink (Pink Opaque Body) Score no score Shape CAPSULE Size 26mm Flavor Imprint Code LU;U43 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70518-2006-0 1 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/09/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203195 04/09/2019 Labeler - REMEDYREPACK INC. (829572556)
Trademark Results [SUPRAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SUPRAX 86164468 4660961 Live/Registered |
Waterbrands, LLC 2014-01-13 |
 SUPRAX 73707543 1500158 Dead/Cancelled |
AMERICAN CYANAMID COMPANY 1988-01-25 |
 SUPRAX 73604097 1456050 Live/Registered |
AMERICAN CYANAMID COMPANY 1986-06-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.