EPHEDRINE SULFATE injection
EPHEDRINE SULFATE by
Drug Labeling and Warnings
EPHEDRINE SULFATE by is a Prescription medication manufactured, distributed, or labeled by Endo USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EPHEDRINE SULFATE INJECTION safely and effectively. See full prescribing information for EPHEDRINE SULFATE INJECTION.
EPHEDRINE SULFATE INJECTION, for intravenous use
Initial U.S. Approval: 2016
INDICATIONS AND USAGE
Ephedrine Sulfate Injection is an alpha- and beta- adrenergic agonist and a norepinephrine-releasing agent that is indicated for the treatment of clinically important hypotension occurring in the setting of anesthesia. (1)
DOSAGE AND ADMINISTRATION
-
Bolus intravenous injection: 5 mg to 10 mg (equivalent to 3.8 to 7.6 mg ephedrine base) as needed, not to exceed 50 mg. (2)
-
Ready to Use formulation. Do not dilute.
DOSAGE FORMS AND STRENGTHS
Injection: 5 mg/mL ephedrine sulfate in a single-dose, 10 mL vial (50 mg/10 mL, equivalent to 38 mg ephedrine base) (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions during treatment: nausea, vomiting, and tachycardia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Endo at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Interactions that Augment Pressor Effect: clonidine, oxytocin and oxytocic drugs, propofol, monoamine oxidase inhibitors (MAOIs), and atropine. Monitor blood pressure. (7)
Interactions that Antagonize the Pressor Effect: Antagonistic effects with α-adrenergic antagonists, β-adrenergic antagonists, reserpine, quinidine, mephentermine. Monitor blood pressure. (7)
Guanethidine: Ephedrine may inhibit the neuron blockage produced by guanethidine, resulting in loss of antihypertensive effectiveness. Monitor blood pressure and adjust the dosage of pressor accordingly.
Rocuronium: Ephedrine may reduce the onset time of neuromuscular blockade when used for intubation with rocuronium if administered simultaneously with anesthetic induction. Be aware of this potential interaction. No treatment or other interventions are needed.
Epidural anesthesia: Ephedrine may decrease the efficacy of epidural blockade by hastening the regression of sensory analgesia. Monitor and treat the patient according to clinical practice.
Theophylline: Concomitant use of ephedrine may increase the frequency of nausea, nervousness, and insomnia. Monitor patient for worsening symptoms and manage symptoms according to clinical practice.
Cardiac glycosides: Giving ephedrine with a cardiac glycoside, such as digitalis, may increase the possibility of arrhythmias. Carefully monitor patients on cardiac glycosides who are also administered ephedrine.
Revised: 4/2024
-
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Instructions
2.2 Dosing for the Treatment of Clinically Important Hypotension in the Setting of Anesthesia
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pressor Effect with Concomitant Oxytocic Drugs
5.2 Tolerance and Tachyphylaxis
5.3 Risk of Hypertension When Used Prophylactically
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Instructions
Ready to Use formulation. Do not dilute.
Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Ephedrine Sulfate Injection is a clear, colorless solution. Do not use if discolored.
2.2 Dosing for the Treatment of Clinically Important Hypotension in the Setting of Anesthesia
The recommended dosages for the treatment of clinically important hypotension in the setting of anesthesia is an initial dose of 5 mg to 10 mg administered by intravenous bolus. Administer additional boluses as needed, not to exceed a total dosage of 50 mg.
Adjust dosage according to the blood pressure goal (i.e., titrate to effect).
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Pressor Effect with Concomitant Oxytocic Drugs
Serious postpartum hypertension has been described in patients who received both a vasopressor (i.e., methoxamine, phenylephrine, ephedrine) and an oxytocic (i.e., methylergonovine, ergonovine) [see Drug Interactions (7)]. Some of these patients experienced a stroke. Carefully monitor the blood pressure of individuals who have received both ephedrine and an oxytocic.
5.2 Tolerance and Tachyphylaxis
Data indicate that repeated administration of ephedrine can result in tachyphylaxis. Clinicians treating anesthesia-induced hypotension with Ephedrine Sulfate Injection should be aware of the possibility of tachyphylaxis and should be prepared with an alternative pressor to mitigate unacceptable responsiveness.
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of ephedrine sulfate were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Gastrointestinal disorders: Nausea, vomiting
Cardiac disorders: Tachycardia, palpitations (thumping heart), reactive hypertension, bradycardia, ventricular ectopics, R-R variability
Nervous system disorders: Dizziness
Psychiatric disorders: Restlessness
-
7 DRUG INTERACTIONS
Interactions that Augment the Pressor Effect Oxytocin and oxytocic drugs Clinical Impact: Serious postpartum hypertension has been described in patients who received both a vasopressor (i.e., methoxamine, phenylephrine, ephedrine) and an oxytocic (i.e., methylergonovine, ergonovine). Some of these patients experienced a stroke. Intervention: Carefully monitor the blood pressure of individuals who have received both ephedrine and an oxytocic. Clonidine, propofol, monoamine oxidase inhibitors (MAOIs), atropine Clinical Impact: These drugs augment the pressor effect of ephedrine. Intervention: Carefully monitor the blood pressure of individuals who have received both ephedrine and any of these drugs. Interactions that Antagonize the Pressor Effect Clinical Impact: These drugs antagonize the pressor effect of ephedrine. Intervention: Carefully monitor the blood pressure of individuals who have received both ephedrine and any of these drugs. Examples: α-adrenergic antagonists, β-adrenergic receptor antagonists, reserpine, quinidine, mephentermine Other Drug Interactions Guanethidine Clinical Impact: Ephedrine may inhibit the neuron blockage produced by guanethidine, resulting in loss of antihypertensive effectiveness. Intervention: Clinician should monitor patient for blood pressor response and adjust the dosage or choice of pressor accordingly. Rocuronium Clinical Impact: Ephedrine may reduce the onset time of neuromuscular blockade when used for intubation with rocuronium if administered simultaneously with anesthetic induction. Intervention: Be aware of this potential interaction. No treatment or other interventions are needed. Epidural anesthesia Clinical Impact: Ephedrine may decrease the efficacy of epidural blockade by hastening the regression of sensory analgesia. Intervention: Monitor and treat the patient according to clinical practice. Theophylline Clinical Impact: Concomitant use of ephedrine may increase the frequency of nausea, nervousness, and insomnia. Intervention: Monitor patient for worsening symptoms and manage symptoms according to clinical practice. Cardiac glycosides Clinical Impact: Giving ephedrine with a cardiac glycoside, such as digitalis, may increase the possibility of arrhythmias. Intervention: Carefully monitor patients on cardiac glycosides who are also administered ephedrine. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from randomized studies, case series, and reports of ephedrine sulfate use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. However, there are clinical considerations (see Clinical Considerations). In animal reproduction studies, decreased fetal survival and fetal body weights were observed in the presence of maternal toxicity after normotensive pregnant rats were administered 60 mg/kg intravenous ephedrine sulfate (12 times the maximum recommended human dose (MRHD) of 50 mg/day). No malformations or embryofetal adverse effects were observed when pregnant rats or rabbits were treated with intravenous bolus doses of ephedrine sulfate during organogenesis at doses 1.9 and 7.7 times the MRHD, respectively (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
Untreated hypotension associated with spinal anesthesia for cesarean section is associated with an increase in maternal nausea and vomiting. A decrease in uterine blood flow due to maternal hypotension may result in fetal bradycardia and acidosis.
Fetal/Neonatal Adverse Reactions
Cases of potential metabolic acidosis in newborns at delivery with maternal ephedrine exposure have been reported in the literature. These reports describe umbilical artery pH of ≤7.2 at the time of delivery [see Clinical Pharmacology (12.3)]. Monitoring of the newborn for signs and symptoms of metabolic acidosis may be required. Monitoring of infant’s acid‐base status is warranted to ensure that an episode of acidosis is acute and reversible.
Data
Animal Data
Decreased fetal body weights were observed when pregnant rats were administered intravenous bolus doses of 60 mg/kg ephedrine sulfate (12 times the maximum recommended human dose (MRHD) of 50 mg based on body surface area) from Gestation Day 6-17. This dose was associated with evidence of maternal toxicity (decreased body weight of dams and abnormal head movements). No malformations or fetal deaths were noted at this dose. No effects on fetal body weight were noted at 10 mg/kg (1.9 times the MRHD of 50 mg).
No evidence of malformations or embryo-fetal toxicity were noted in pregnant rabbits administered intravenous bolus doses up to 20 mg/kg ephedrine sulfate (7.7 times the maximum recommended human dose (MRHD) of 50 mg based on body surface area) from Gestation Day 6-20. This dose was associated with expected pharmacological maternal effects (increased respiration rate, dilated pupils, piloerection).
Decreased fetal survival and body weights in the presence of maternal toxicity (increased mortality) were noted when pregnant dams were administered intravenous bolus doses of 60 mg/kg epinephrine sulfate (approximately 12 times the MRHD based on body surface area) from GD 6 through Lactation Day 20. No adverse effects were noted at 10 mg/kg (1.9 times the MRHD).
8.2 Lactation
Risk Summary
A single published case report indicates that ephedrine is present in human milk. However, no information is available on the effects of the drug on the breastfed infant or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Ephedrine Sulfate Injection and any potential adverse effects on the breastfed child from Ephedrine Sulfate Injection or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Animal Toxicity Data
In a study in which juvenile rats were administered intravenous bolus doses of 2, 10, or 60 mg/kg ephedrine sulfate daily from Postnatal Day 35 to 56, an increased incidence of mortality was noted at the high dose of 60 mg/kg. The no-adverse-effect level was 10 mg/kg (approximately 1.9 times a maximum daily dose of 50 mg in a 60 kg person based on body surface area).
8.5 Geriatric Use
Clinical studies of ephedrine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
Ephedrine and its metabolite are excreted in urine. In patients with renal impairment, excretion of ephedrine is likely to be affected with a corresponding increase in elimination half-life, which will lead to slow elimination of ephedrine and consequently prolonged pharmacological effect and potentially adverse reactions. Monitor patients with renal impairment carefully after the initial bolus dose for adverse events.
- 10 OVERDOSAGE
-
11 DESCRIPTION
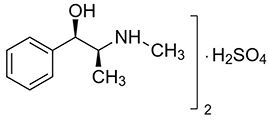
Ephedrine is an alpha- and beta-adrenergic agonist and a norepinephrine-releasing agent. Ephedrine Sulfate Injection, 5 mg/mL is a clear, colorless, sterile, ready-to-use solution for intravenous injection. The chemical name of ephedrine sulfate is (1R,2S)-(-)-2-methylamine-1-phenylpropan-1-ol sulfate, and the molecular weight is 428.5 g/mol. Its molecular formula is (C10H15NO)2 · H2SO4 and its structural formula is depicted below:
Ephedrine sulfate is freely soluble in water and ethanol, very slightly soluble in chloroform, and practically insoluble in ether. Each mL contains ephedrine sulfate 5 mg (equivalent to 3.8 mg ephedrine base), sodium chloride 9 mg, and sodium hydroxide and/or acetic acid for pH adjustment, if necessary. The pH range is 4.5 to 7.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ephedrine sulfate is a sympathomimetic amine that directly acts as an agonist at α- and β-adrenergic receptors and indirectly causes the release of norepinephrine from sympathetic neurons. Pressor effects by direct alpha- and beta-adrenergic receptor activation are mediated by increases in arterial pressures, cardiac output, and peripheral resistance. Indirect adrenergic stimulation is caused by norepinephrine release from sympathetic nerves.
12.2 Pharmacodynamics
Ephedrine stimulates heart rate and cardiac output and variably increases peripheral resistance; as a result, ephedrine usually increases blood pressure. Stimulation of the α-adrenergic receptors of smooth muscle cells in the bladder base may increase the resistance to the outflow of urine. Activation of β-adrenergic receptors in the lungs promotes bronchodilation.
The overall cardiovascular effect from ephedrine is the result of a balance among α-1 adrenoceptor-mediated vasoconstriction, β-2 adrenoceptor-mediated vasoconstriction, and β-2 adrenoceptor-mediated vasodilatation. Stimulation of the β-1 adrenoceptors results in positive inotrope and chronotrope action.
Tachyphylaxis to the pressor effects of ephedrine may occur with repeated administration [see Warnings and Precautions 5.2].
12.3 Pharmacokinetics
Publications studying pharmacokinetics of oral administration of (-)-ephedrine support that (‑)‑ephedrine is metabolized into norephedrine. However, the metabolism pathway is unknown. Both the parent drug and the metabolite are excreted in urine. Limited data after IV administration of ephedrine support similar observations of urinary excretion of drug and metabolite. The plasma elimination half-life of ephedrine following oral administration was about 6 hours.
Ephedrine crosses the placental barrier [see Use in Specific Populations 8.1].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Two-year feeding studies in rats and mice conducted under the National Toxicology Program (NTP) demonstrated no evidence of carcinogenic potential with ephedrine sulfate at doses up to 10 mg/kg/day and 27 mg/kg/day (approximately 2 times and 3 times the maximum human recommended dose on a mg/m2 basis, respectively).
Mutagenesis: Ephedrine sulfate tested negative in the in vitro bacterial reverse mutation assay, the in vitro mouse lymphoma assay, the in vitro sister chromatid exchange, the in vitro chromosomal aberration assay, and the in vivo rat bone marrow micronucleus assay.
Impairment of Fertility: There was no impact on fertility or early embryonic development in a study in which male rats were administered intravenous bolus doses of 0, 2, 10, or 60 mg/kg ephedrine sulfate (up to 12 times the maximum recommended human dose of 50 mg based on body surface area) for 28 days prior to mating and through gestation and females were treated for 14 days prior to mating through Gestation Day 7.
-
14 CLINICAL STUDIES
The evidence for the efficacy of ephedrine injection is derived from the published literature. Increases in blood pressure following administration of ephedrine were observed in 14 studies, including 9 where ephedrine was used in pregnant women undergoing neuraxial anesthesia during Cesarean delivery, 1 study in non-obstetric surgery under neuraxial anesthesia, and 4 studies in patients undergoing surgery under general anesthesia. Ephedrine has been shown to raise systolic and mean blood pressure when administered as a bolus dose following the development of hypotension during anesthesia.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
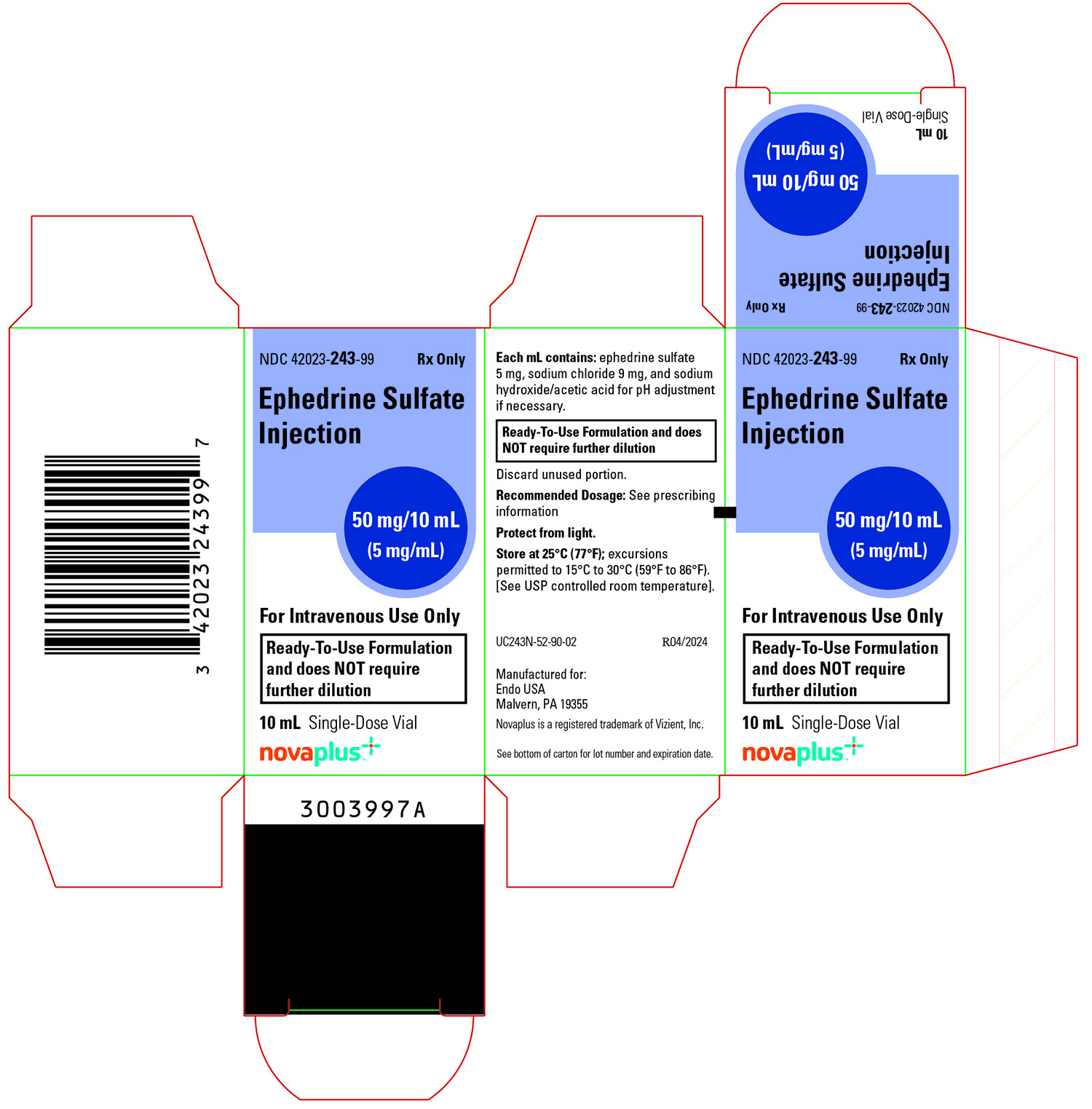
Ephedrine Sulfate Injection 50 mg/10 mL (5 mg/mL, equivalent to 3.8 mg/mL ephedrine base), is a clear, colorless solution and is supplied as follows:
NDC Strength How Supplied 42023-243-99 50 mg/10 mL (5 mg/mL) 10 mL clear, glass, single-dose vial; (supplied in packages of 1) Vial stoppers are not manufactured with natural rubber latex. Store Ephedrine Sulfate Injection, 5 mg/mL at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Store in carton until time of use. For single use only. Discard unused portion.
Manufactured for:
Endo USA
Malvern, PA 19355
© 2024 Endo, Inc. or one of its affiliates.

Novaplus is a registered trademark of Vizient, Inc.
04/2024
- PRINCIPAL DISPLAY PANEL - 1 X 10 mL Single-Dose Vial Carton
-
INGREDIENTS AND APPEARANCE
EPHEDRINE SULFATE
ephedrine sulfate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42023-243 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPHEDRINE SULFATE (UNII: U6X61U5ZEG) (EPHEDRINE - UNII:GN83C131XS) EPHEDRINE SULFATE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL ACETIC ACID (UNII: Q40Q9N063P) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42023-243-99 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 01/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213994 01/31/2024 Labeler - Endo USA, Inc. (119185057) Registrant - Endo USA, Inc. (119185057)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.