DOCTOR HAND FRESS Gel by C-MAX KOREA CO.,LTD. Drug Facts
DOCTOR HAND FRESS Gel by
Drug Labeling and Warnings
DOCTOR HAND FRESS Gel by is a Otc medication manufactured, distributed, or labeled by C-MAX KOREA CO.,LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DOCTOR HAND FRESS GEL- alcohol gel
C-MAX KOREA CO.,LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
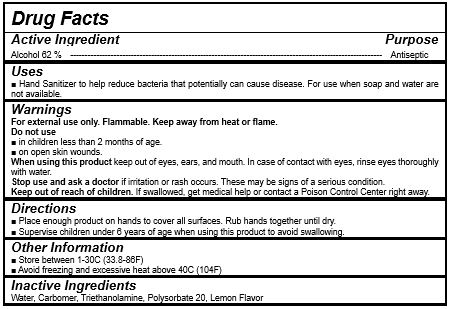
Drug Facts
Antiseptic
instant healthcare personnel hand antiseptic to reduce bacteria that potentially can cause disease
instant hand antiseptic to decrease bacteria on the skin
recommended for repeated use
Apply to clean, dry hands. Apply sufficient amount to thoroughly wet all surfaces of hands and fingers. Rub onto hands until dry.
Supervise children in the use of this product.
For external use only.
Flammable, keep away from fire or flame.
When using this product keep out of eyes. If contact with eyes occurs, rinse promptly and thoroughly with water.
Stop use and ask a doctor if significant irritation or sensitization develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| DOCTOR HAND FRESS GEL
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - C-MAX KOREA CO.,LTD. (557821673) |
| Registrant - C-MAX KOREA CO.,LTD. (557821673) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| C-MAX KOREA CO.,LTD. | 557821673 | manufacture(70949-001) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.