005 Curad Saline Laxative
Curad Saline Laxative by
Drug Labeling and Warnings
Curad Saline Laxative by is a Otc medication manufactured, distributed, or labeled by Medline Industries, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CURAD SALINE LAXATIVE- sodium phosphate enema
Medline Industries, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
005 Curad Saline Laxative
Active ingredient
(in each 118 mL delivered dose)
Dibasic sodium phosphate 7 g
Monobasic sodium phosphate 19 g
Warnings
For rectal use only
Ask a doctor before use if you have
- a sodium-restricted diet
- abdominal pain, nausea or vomiting
- kidney disease, heart problems, or are dehydrated
- noticed a sudden change in bowel habits that lasts over 2 weeks
Ask a doctor or pharmacist before use if you are
taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
When using this product
- do not use more than directed. Serious side effects may occur from excess dosage.
- do not use for more than 3 days
Directions
Do not use more unless directed by a doctor. See Warnings.
|
adults & children 12 years and over |
one bottle once daily |
|
children 2 to under 12 years |
1/2 bottle once daily Discard unused portion |
|
children under 2 years |
do not use |
CAUTION:
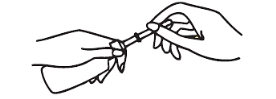
Remove green protective shield before inserting. Hold bottle upright, grasping bottle cap with fingers. Grasp protective shield with other hand and pull gently to remove.
Positioning:
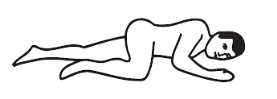
Left-side position: lie on left side with knee bent and arms at rest.
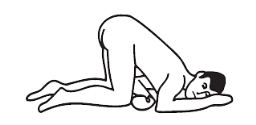
Knee-chest position: kneel, then lower head and chest forward until left side of face is resting on surface. Position arms comfortably.
Administering enema:
- with steady pressure, gently insert enema with tip pointing toward navel.
- squeeze bottle until recommended dose is expelled (it is not necessary to empty unit completely. Bottle contains more liquid than needed for effective use. A small amount of liquid will remain in bottle after squeezing).
- remove tip from rectum
- stop using if tip is hard to insert. Forcing the tip into the rectum can cause injury (especially if you have hemorrhoids). If enema tip causes rectal bleeding or pain, get immediate medical care.
- maintain position until urge to evacuate is strong (usually 1 to 5 minutes)
- if no urge is felt after 5 minutes of using, try to empty bowel. Call a doctor promptly if no liquid comes out of the rectum after 30 minutes because dehydration could occur.
Other Information
- each 118 mL contains: sodium 4.3 g
- store at room temperature 20-25 deg C (68-77 deg F)
- this product generally produces bowel movement in 1 to 5 minutes
Side and Principal Display Panel - box
NDC: 53329-005-11
READY-TO-USE
ENEMA
SALINE LAXATIVE
- For Relief of Occasional Constipation
- Soft, flexible comfort tip
- Latex free
4.5 Fl OZ (133 ml)
| CURAD SALINE LAXATIVE
sodium phosphate enema |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Medline Industries, Inc. (025460908) |