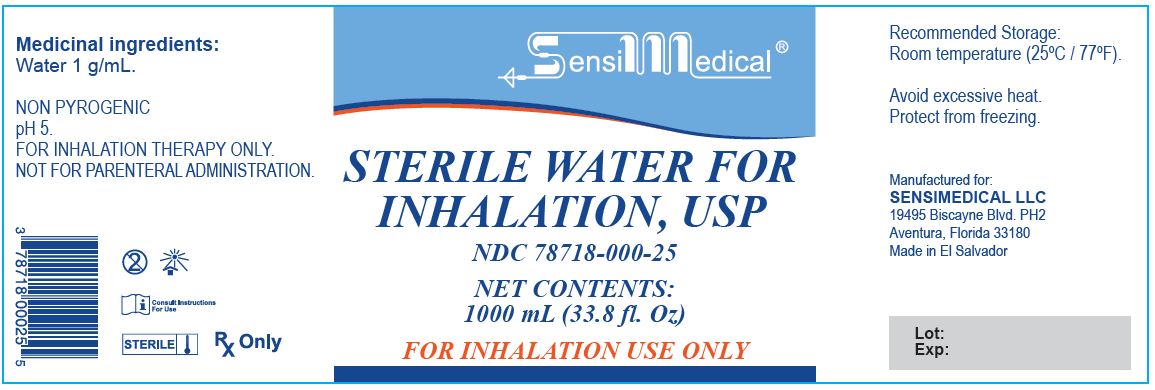
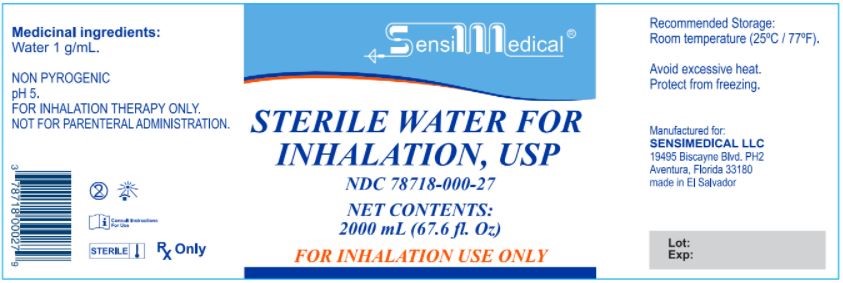
Sterile Water for Inhalation
Sterile Water for INHALATION by
Drug Labeling and Warnings
Sterile Water for INHALATION by is a Prescription medication manufactured, distributed, or labeled by SENSIMEDICAL LLC, Laboratorios Biogalenic. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
STERILE WATER FOR INHALATION- sterile water for inhalation inhalant
SENSIMEDICAL LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Sterile Water for Inhalation
| STERILE WATER FOR INHALATION
sterile water for inhalation inhalant |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SENSIMEDICAL LLC (117526048) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Laboratorios Biogalenic | 851259507 | manufacture(78718-000) , api manufacture(78718-000) | |
Revised: 2/2022
Document Id: a3d0e6c8-2b71-4453-a6b6-1665be77ab11
Set id: a2685f47-7f67-47b2-b4c1-6de587b768a7
Version: 4
Effective Time: 20220216
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.