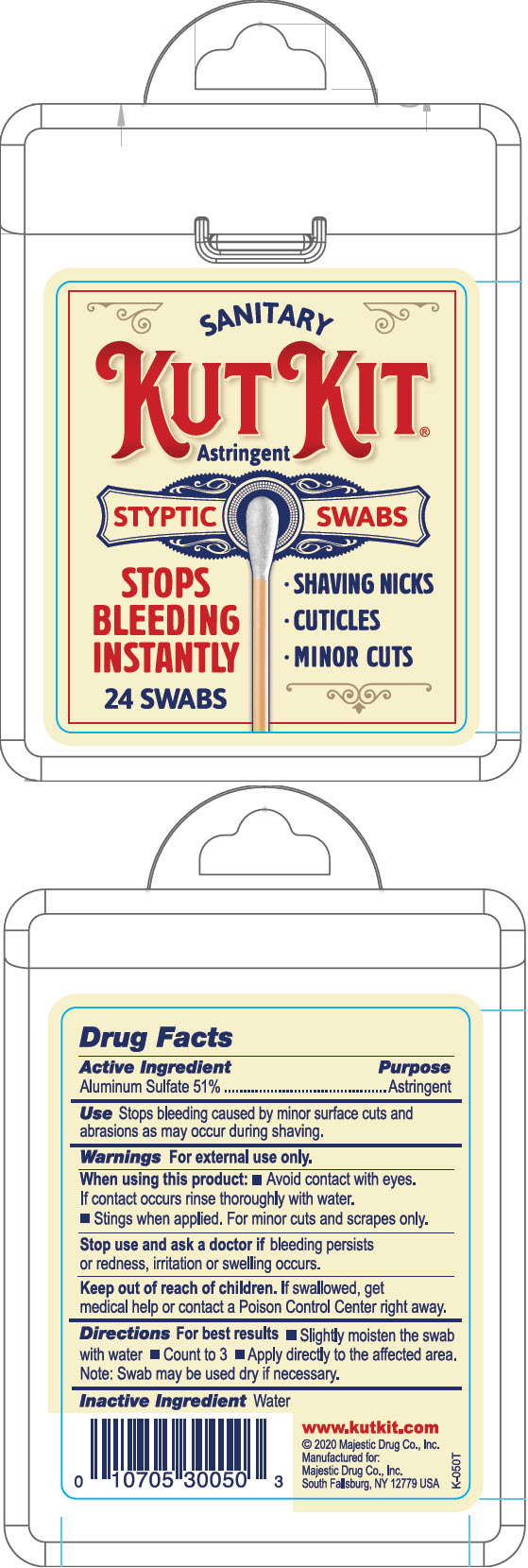
KutKit by Majestic Drug Co., Inc. KutKit®
KutKit by
Drug Labeling and Warnings
KutKit by is a Otc medication manufactured, distributed, or labeled by Majestic Drug Co., Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
KUTKIT- aluminum sulfate swab
Majestic Drug Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
KutKit®
Warnings
For external use only.
| KUTKIT
aluminum sulfate swab |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Majestic Drug Co., Inc. (001496777) |
Revised: 1/2022
Document Id: 635fb007-7807-4757-a275-7ac37e67d72c
Set id: a29460b5-7dd2-479d-9b4b-5ab8dc135ad4
Version: 3
Effective Time: 20220124
Trademark Results [KutKit]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KUTKIT 78759582 3158380 Live/Registered |
Majestic Drug Co., Inc. 2005-11-22 |
 KUTKIT 75564491 2365413 Dead/Cancelled |
Majestic Drug Co., Inc. 1998-10-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.