BD PERSIST- povidone-iodine, alcohol solution
BD PERSIST by
Drug Labeling and Warnings
BD PERSIST by is a Otc medication manufactured, distributed, or labeled by Becton Dickinson and Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
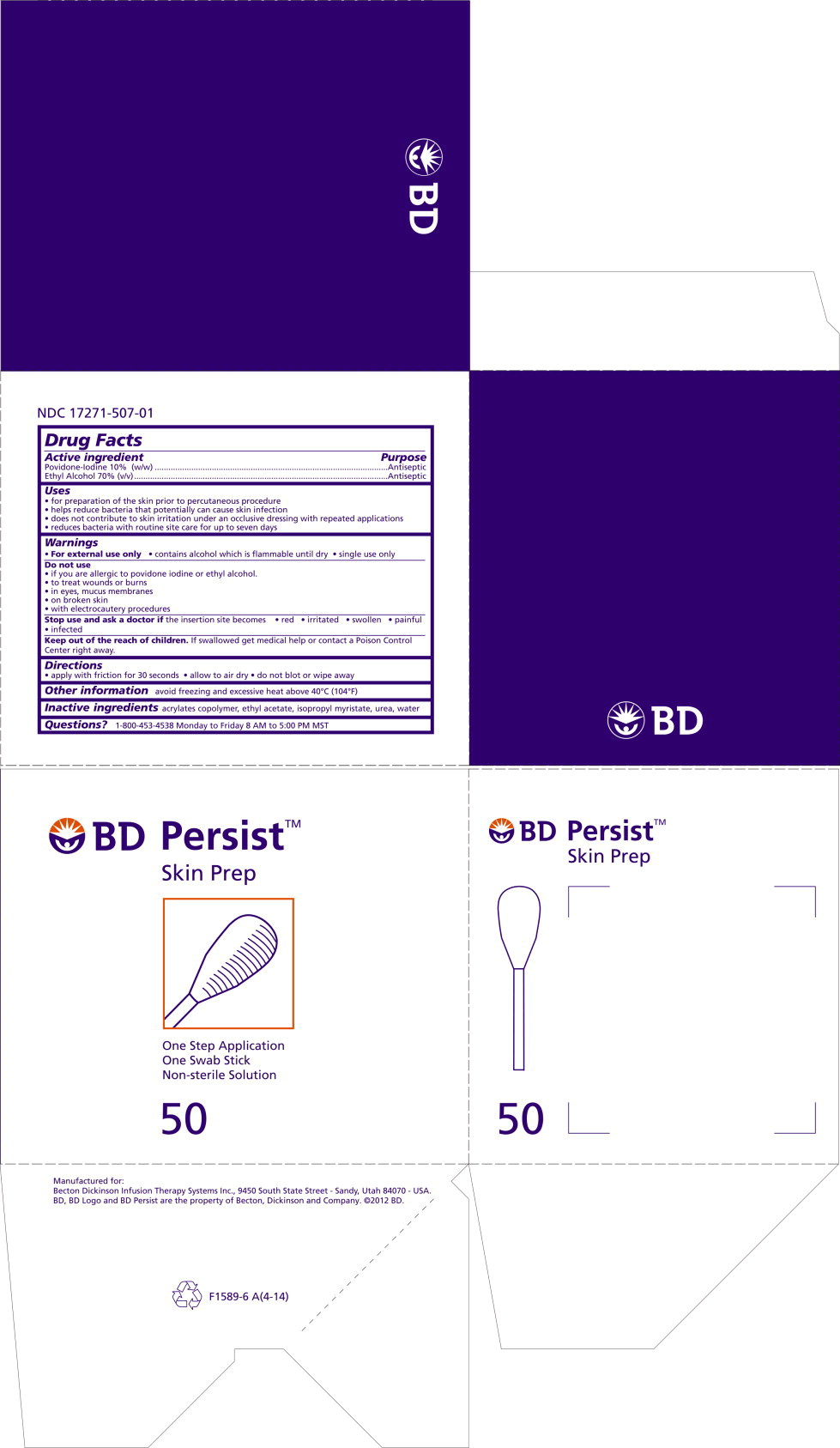
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Package Label
BD Persist™ Skin Prep
1 Swab Stick Non-sterile solution REF 386401 NDC: 17271-507-01 -
INGREDIENTS AND APPEARANCE
BD PERSIST
povidone-iodine, alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 17271-507 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength povidone-iodine (UNII: 85H0HZU99M) (iodine - UNII:9679TC07X4) iodine 85 mg in 1 mL alcohol (UNII: 3K9958V90M) (alcohol - UNII:3K9958V90M) alcohol 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength carbomer copolymer Type A (UNII: 71DD5V995L) ethyl acetate (UNII: 76845O8NMZ) isopropyl myristate (UNII: 0RE8K4LNJS) urea (UNII: 8W8T17847W) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17271-507-01 50 in 1 CARTON 02/21/1996 1 2 mL in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC: 17271-507-02 25 in 1 CARTON 02/21/1996 2 7 mL in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 02/21/1996 Labeler - Becton, Dickinson and Company (124987988) Establishment Name Address ID/FEI Business Operations Becton, Dickinson and Company 124987988 MANUFACTURE(17271-507)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.