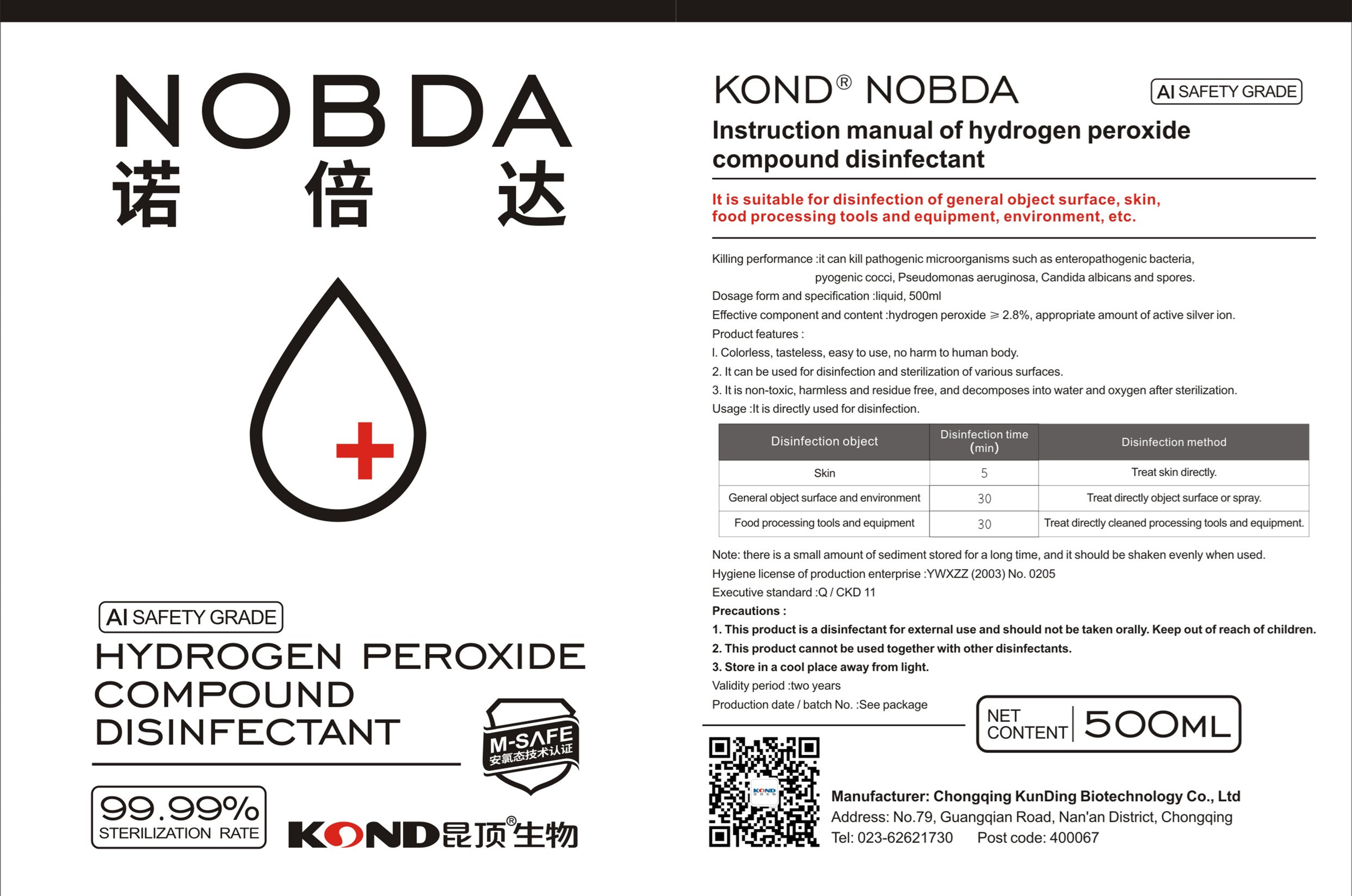
Nobda Hydrogen peroxide compound disinfectant by Chongqing Kunding Biologic Technology Co., Ltd.
Nobda Hydrogen peroxide compound disinfectant by
Drug Labeling and Warnings
Nobda Hydrogen peroxide compound disinfectant by is a Otc medication manufactured, distributed, or labeled by Chongqing Kunding Biologic Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NOBDA HYDROGEN PEROXIDE COMPOUND DISINFECTANT- hydrogen peroxide compound disinfectan liquid
Chongqing Kunding Biologic Technology Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
it can kill pathogenic microorganisms such as enteropathogenic bacteria,pyogenic cocci, Pseudomonas aeruginosa, Candida albicans and spores.
| NOBDA HYDROGEN PEROXIDE COMPOUND DISINFECTANT
hydrogen peroxide compound disinfectan liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Chongqing Kunding Biologic Technology Co., Ltd. (545203072) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Chongqing Kunding Biologic Technology Co., Ltd. | 545203072 | manufacture(54520-002) | |
Revised: 4/2020
Document Id: a2d7b93f-09d8-746a-e053-2995a90ae0c3
Set id: a2b4705f-682e-95b5-e053-2a95a90a66d5
Version: 2
Effective Time: 20200409
Chongqing Kunding Biologic Technology Co., Ltd.