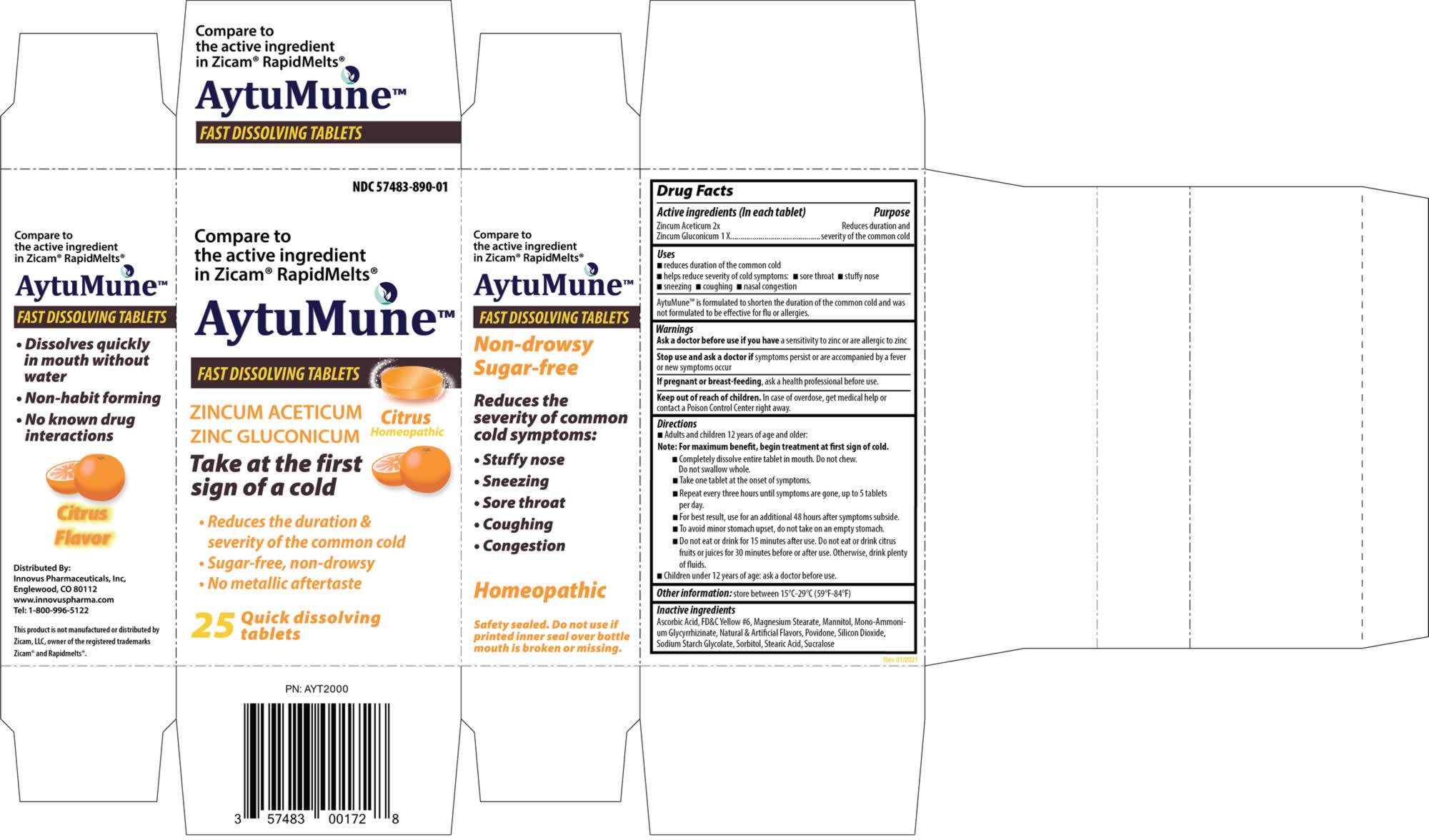
AYTUMUNE Fast Dissolving Tablets
AytuMune by
Drug Labeling and Warnings
AytuMune by is a Homeopathic medication manufactured, distributed, or labeled by Innovus Pharmaceuticals, Inc., Dynamic Pharmaceuticals, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AYTUMUNE- zinc acetate anhydrous and zinc gluconate tablet
Innovus Pharmaceuticals, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
AYTUMUNE Fast Dissolving Tablets
USES
- reduces duration of the common cold
- helps reduce severity of cold symptoms:
- sore throat
- stuffy nose
- sneezing
- coughing
- nasal congestion
AytuMune is formulated to shorten the duration of the common cold and was not formulated to be effective for flu or allergies.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Adults and children 12 years of age and older:
Note : For maximum benefit, begin treatment at first sign of cold.
- Completely dissolve entire tablet in mouth. Do not chew. Do not swallow whole.
- Take one tablet at the onset of symptoms.
- Repeat every three hours until symptoms are gone, up to 5 tablets per day.
- For best result, use for an additional 48 hours after symptoms subside.
- To avoid minor stomach upset, do not take on an empty stomach,
- Do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
Children under 12 years of age: ask a doctor before use.
| AYTUMUNE
zinc acetate anhydrous and zinc gluconate tablet |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Innovus Pharmaceuticals, Inc. (962507187) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dynamic Pharmaceuticals, Inc | 617660712 | manufacture(57483-890) | |
Trademark Results [AytuMune]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AYTUMUNE 90160351 not registered Live/Pending |
Innovus Pharmaceuticals, Inc. 2020-09-04 |
 AYTUMUNE 90160190 not registered Live/Pending |
Innovus Pharmaceuticals, Inc. 2020-09-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.