HYMPAVZI- marstacimab-hncq injection, solution
HYMPAVZI by
Drug Labeling and Warnings
HYMPAVZI by is a Prescription medication manufactured, distributed, or labeled by Pfizer Laboratories Div Pfizer Inc, Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC, Pfizer Manufacturing Belgium NV, Pfizer Ireland Pharmaceuticals Unlimited Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HYMPAVZI safely and effectively. See full prescribing information for HYMPAVZI.
HYMPAVZI (marstacimab-hncq) injection, for subcutaneous use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
HYMPAVZI is a tissue factor pathway inhibitor (TFPI) antagonist indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with:
- hemophilia A (congenital factor VIII deficiency) without factor VIII inhibitors, or
- hemophilia B (congenital factor IX deficiency) without factor IX inhibitors. (1)
DOSAGE AND ADMINISTRATION
- See Full Prescribing Information for important dosing and administration instructions. (2.1, 2.2, 2.3, 2.4, 2.5, 2.6)
-
The recommended dosage of HYMPAVZI is:
- o Loading dose: 300 mg (two 150 mg injections) by subcutaneous injection
- o Maintenance dose: One week after the loading dose, initiate maintenance dosing of 150 mg every week by subcutaneous injection on the same day each week, at any time of day. (2.1)
- Dose adjustment to 300 mg subcutaneous injection weekly can be considered. (2.1)
- Factor VIII and factor IX products can be administered for the treatment of breakthrough bleeds in patients receiving HYMPAVZI. Do not use additional doses of HYMPAVZI to treat breakthrough bleeds. (2.4)
- Temporarily discontinue HYMPAVZI before major surgery. (2.5)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Thromboembolic Events: Thromboembolic events may occur. Interrupt HYMPAVZI prophylaxis if symptoms occur. (5.1)
- Hypersensitivity: Hypersensitivity reactions may occur. In the event of a severe allergic reaction, discontinue HYMPAVZI. (5.2)
- Embryofetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. (5.3, 8.1, 8.3)
ADVERSE REACTIONS
Adverse reactions reported in ≥3% of HYMPAVZI-treated patients were injection site reaction, headache, and pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Administration
2.3 Changing to HYMPAVZI
2.4 Guidance on Use with Breakthrough Bleed Treatments
2.5 Temporary Interruption for Surgery and Other Interventions
2.6 Pregnancy Testing
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Events
5.2 Hypersensitivity
5.3 Embryofetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hemophilia A without FVIII Inhibitors or Hemophilia B without FIX Inhibitors
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Recommended Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
HYMPAVZI is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with:
- hemophilia A (congenital factor VIII deficiency) without factor VIII inhibitors, or
- hemophilia B (congenital factor IX deficiency) without factor IX inhibitors.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
For subcutaneous use only.
The recommended dosage of HYMPAVZI for adult and pediatric patients 12 years of age and older is as follows:Loading Dose
300 mg (two 150 mg subcutaneous injections)
If more than one injection is required to deliver a complete dose, administer each injection at a different injection site.Maintenance Dose
One week after the loading dose, initiate maintenance dosing of 150 mg every week by subcutaneous injection on the same day each week, at any time of day.Dose Adjustment During Treatment
Consider a dose adjustment to 300 mg subcutaneous injection weekly in patients weighing greater than or equal to 50 kg when control of bleeding events is judged to be inadequate by the healthcare provider. Safety and efficacy of HYMPAVZI at doses above 300 mg weekly have not been established.If more than one injection is required to deliver a complete dose, administer each injection at a different injection site.
Missed Doses
For patients on a maintenance dose of 150 mg:
If a dose is missed, administer as soon as possible before the day of the next scheduled dose, and then resume usual 150 mg subcutaneous weekly dosing schedule (same schedule as prior to the missed dose or new schedule based on date of administration of missed dose).If more than 13 days have passed since the last dose was administered, administer a loading dose of 300 mg by subcutaneous injection followed by a resumption of 150 mg by subcutaneous injection once weekly thereafter.
For patients on a maintenance dose of 300 mg:
If one or more doses are missed, administer a dose as soon as possible, and then resume 300 mg subcutaneous weekly dosing schedule (same schedule as prior to the missed dose or new schedule based on date of administration of missed dose).2.2 Preparation and Administration
- HYMPAVZI is intended for use under the guidance of a healthcare provider. After proper training in subcutaneous injection technique, a patient may self-inject or the patient’s caregiver may administer HYMPAVZI, if a healthcare provider determines that it is appropriate.
- Refer to the Instructions for Use for complete preparation and administration instructions.
- Prior to subcutaneous administration, HYMPAVZI may be removed from the refrigerator and allowed to warm at room temperature [up to 86°F (30°C)] in the carton for 15 to 30 minutes protected from direct sunlight. Do not warm by using a heat source such as hot water or a microwave. After removal of HYMPAVZI from the refrigerator, use within 7 days or discard [see How Supplied/Storage and Handling (16)].
- Administer HYMPAVZI by subcutaneous injection, once weekly, at any time of the day in the abdomen or front of thigh. Other injection sites are acceptable if required. Administration of HYMPAVZI in the back of upper arm (prefilled syringe only) or buttocks (prefilled pen only) should be performed by a caregiver or healthcare professional only. HYMPAVZI should not be administered into bony areas or areas where the skin is bruised, red, tender or hard, or areas where there are scars or stretch marks. HYMPAVZI should not be injected into a vein. Rotate the injection site with each new injection.
- During treatment with HYMPAVZI, other medicinal products for subcutaneous administration should, preferably, be injected at different anatomical sites.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. HYMPAVZI is a clear and colorless to light yellow solution. Do not use if the solution is cloudy, dark yellow, or contains flakes or particles.
2.3 Changing to HYMPAVZI
Changing from prophylactic factor replacement therapy to HYMPAVZI: Prior to initiation of HYMPAVZI, discontinue treatment with clotting factor concentrates (factor VIII or factor IX concentrates). HYMPAVZI can be initiated at any time after discontinuing clotting factor concentrates.
No data are available in patients changing from non-factor-based hemophilia medicinal products to HYMPAVZI.
2.4 Guidance on Use with Breakthrough Bleed Treatments
Factor VIII and factor IX products can be administered for the treatment of breakthrough bleeds in patients receiving HYMPAVZI. Do not use additional doses of HYMPAVZI to treat breakthrough bleeds. Healthcare providers should discuss with all patients and/or caregivers the dose and schedule of clotting factor concentrates to use, if required, while receiving HYMPAVZI prophylaxis, including using the lowest possible effective dose of clotting factor concentrate [see Warnings and Precautions (5.1)]. Please refer to the Full Prescribing Information for the clotting factor concentrate being used.
2.5 Temporary Interruption for Surgery and Other Interventions
Management in the Perioperative Setting
HYMPAVZI has not been evaluated in the setting of major surgery. Patients have had minor surgical procedures without discontinuing HYMPAVZI prophylaxis in clinical studies.For major surgery, discontinue HYMPAVZI and initiate management per local standard of care with clotting factor concentrate and measures to manage the risk of venous thrombosis which can be elevated in the perioperative period. Consult the product information for the clotting factor concentrate for dosage guidelines in patients with hemophilia undergoing major surgery. Resumption of HYMPAVZI therapy should consider the overall clinical status of the patient, including the presence of post-surgical thromboembolic risk factors, use of other hemostatic products and other concomitant medications [see Dosage and Administration (2.1)].
Management in Patients with Acute Severe Illness
There is limited experience with the use of HYMPAVZI in patients with acute severe illness. Reasons to consider temporary dose interruption of HYMPAVZI include occurrence of acute severe illness (e.g., serious infection, sepsis, trauma) in which there may be increased activation of coagulation and which the healthcare provider considers could increase the risks associated with HYMPAVZI administration. Treatment of acute severe illness should be managed per local standard of care, and continued treatment with HYMPAVZI in this situation should be weighed against the potential risks involved. Resume HYMPAVZI therapy once patient has clinically recovered [see Dosage and Administration (2.1)].2.6 Pregnancy Testing
Verify that females of reproductive potential are not pregnant prior to initiating HYMPAVZI [see Warnings and Precautions (5.3), Use in Specific Populations (8.1, 8.3)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Events
HYMPAVZI is a tissue factor pathway inhibitor (TFPI) antagonist, and may increase the risk of thromboembolic complications. Venous thrombotic events were reported in clinical studies with HYMPAVZI [see Adverse Reactions (6.1)].
Patients with independent risk factors for thromboembolic events may be at increased risk of thromboembolic events with use of HYMPAVZI.
HYMPAVZI has not been studied in patients with a history of previous thromboembolic events [see Clinical Studies (14.1)]. Consider the benefit and risk of using HYMPAVZI in patients with known risk factors for thromboembolism.
Interrupt HYMPAVZI prophylaxis if diagnostic findings consistent with thromboembolism occur and manage as clinically indicated.
If factor VIII or factor IX products are indicated in a patient receiving HYMPAVZI prophylaxis, the minimum effective dose of factor VIII or factor IX according to the product label is recommended [see Dosage and Administration (2.4)].
5.2 Hypersensitivity
HYMPAVZI may cause hypersensitivity reactions (including, but not limited to urticaria and pruritus). If HYMPAVZI-treated patients develop a severe hypersensitivity reaction, advise patients to discontinue HYMPAVZI and seek immediate emergency treatment.
5.3 Embryofetal Toxicity
Based on its mechanism of action, HYMPAVZI may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with HYMPAVZI and for 2 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Thromboembolic Events [see Warnings and Precautions (5.1)]
- Hypersensitivity [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of HYMPAVZI was evaluated in adolescent and adult patients with severe hemophilia A or B without inhibitors (coagulation factor activity <1%) enrolled in the BASIS study [see Clinical Studies (14.1)]. Patients (N = 116) received HYMPAVZI prophylaxis 300 mg loading dose followed by 150 mg every week starting at Day 8 administered subcutaneously. Among patients receiving HYMPAVZI, 97% were exposed for 6 months or longer and 75% were exposed for at least 1 year.
A serious adverse reaction of peripheral swelling occurred in one patient.
Table 1 summarizes the adverse reactions reported in ≥3% of patients who received HYMPAVZI prophylaxis.
Table 1. Adverse Reactions Reported in ≥3% of Patients Treated with HYMPAVZI* - * During BASIS trial 12-month active treatment phase
Adverse Reaction
Number of Patients
n (%)
(N = 116)
Injection site reaction
11 (9)
Headache
8 (7)
Pruritus
4 (3)
A serious adverse reaction of venous thrombosis occurred in 0.9% of patients (1/116) treated with HYMPAVZI in the open-label extension study [see Clinical Studies (14.1)].
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, HYMPAVZI may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on HYMPAVZI use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Female animal reproduction studies have not been conducted with HYMPAVZI. Although there are no data on marstacimab‑hncq, monoclonal antibodies can be actively transported across the placenta, and marstacimab‑hncq may cause fetal harm.The background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of marstacimab‑hncq in either human or animal milk, the effects on the breastfed child, or the effects on milk production.Endogenous maternal IgG and monoclonal antibodies are known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to marstacimab‑hncq are unknown.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for HYMPAVZI and any potential adverse effects on the breastfed infant from HYMPAVZI or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
HYMPAVZI may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating HYMPAVZI treatment.Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with HYMPAVZI and for 2 months after the last dose.8.4 Pediatric Use
The safety and effectiveness of HYMPAVZI to prevent or reduce the frequency of bleeding episodes in hemophilia A or B without inhibitors have been established in pediatric patients aged 12 years and older [see Clinical Studies (14.1)]. Use of HYMPAVZI for this indication is supported by evidence from an open‑label, multi‑center phase 3 study in 19 adolescents and 97 adults with hemophilia without inhibitors.
The safety and effectiveness of HYMPAVZI have not been established in pediatric patients younger than 12 years old.
8.5 Geriatric Use
One patient 65 years of age and older was enrolled in the clinical studies for hemophilia A or B without inhibitors [see Clinical Studies (14.1)]. Clinical studies of HYMPAVZI did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects.
-
11 DESCRIPTION
Marstacimab‑hncq is a tissue factor pathway inhibitor (TFPI) antagonist, human monoclonal immunoglobulin G Type 1 (IgG1) antibody. Marstacimab‑hncq is produced by Chinese hamster ovary (CHO) cells by recombinant DNA technology and has a molecular mass of approximately 146 kDa.
HYMPAVZI (marstacimab‑hncq) injection is supplied as a sterile, preservative-free solution for subcutaneous administration. The drug product is supplied as either a single-dose 150 mg/mL prefilled syringe or as a single‑dose 150 mg/mL prefilled pen. The solution of marstacimab‑hncq is clear and colorless to light yellow with a pH of 5.8.
Each 150 mg/mL prefilled syringe or prefilled pen delivers 1 mL of HYMPAVZI. Each 1 mL of HYMPAVZI contains 150 mg of marstacimab‑hncq, and the inactive ingredients edetate disodium (0.05 mg), histidine (1.12 mg), L-histidine monohydrochloride (2.67 mg), polysorbate 80 (0.2 mg), and sucrose (85 mg), in Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Marstacimab‑hncq is a human monoclonal IgG1 antibody directed against the Kunitz domain 2 (K2) of TFPI to neutralize TFPI activity and enhance coagulation. TFPI is the primary inhibitor of the extrinsic coagulation cascade and negatively regulates thrombin generation within the extrinsic pathway of coagulation by inactivating the protease functions of FXa/FVIIa/TF complex. TFPI binds to and inhibits the factor Xa active site via its second Kunitz inhibitor domain (K2).
12.2 Pharmacodynamics
Marstacimab‑hncq causes an increase in total TFPI (comprised of free TFPI and TFPI bound to marstacimab) and downstream biomarkers of thrombin generation such as prothrombin fragments 1+2, peak thrombin, and D‑Dimer in patients with hemophilia. These changes were observed and persisted over a 7-day period following a single subcutaneous dose and were reversible after treatment discontinuation.
12.3 Pharmacokinetics
Estimated mean marstacimab‑hncq Cmin,ss, Cmax,ss, and Cavg,ss for adults and adolescents weighing at least 35 kg following marstacimab‑hncq 150 mg subcutaneous once-weekly administration are shown in Table 2. Marstacimab‑hncq area under the plasma concentration-time curve (AUC) and maximum plasma concentration (Cmax) increase in a greater than dose-proportional manner over the dose range of 100 mg to 450 mg (0.67 to 3 times the approved recommended dosage).
Mean steady-state accumulation ratio for marstacimab‑hncq is approximately 4 to 5. Marstacimab‑hncq steady‑state concentrations are achieved by approximately 60 days (8th or 9th subcutaneous dose) when administered once weekly.
Table 2. Steady-State Marstacimab‑hncq Plasma Concentrations Following Once-Weekly Subcutaneous Administration of 150 mg (with a Loading Dose of 300 mg Subcutaneous) Data are presented as arithmetic mean (%CV). Cmin,ss = minimum plasma concentration at steady state; Cmax,ss = maximum plasma concentration at steady state; Cavg,ss = average plasma concentration at steady state. Parameter
Adults
Adolescents
Cmin,ss (mcg/mL)
13.7 (90.4%)
27.3 (53.2%)
Cmax,ss (mcg/mL)
17.9 (77.5%)
34.7 (48.5%)
Cavg,ss (mcg/mL)
16.5 (81.2%)
32.1 (49.5%)
Absorption
Bioavailability of marstacimab‑hncq following subcutaneous administration is approximately 71%. Median Tmax ranges from 23 to 59 hours following multiple subcutaneous administrations of marstacimab‑hncq to patients with hemophilia. No clinically significant differences were seen in marstacimab‑hncq bioavailability when administered subcutaneously in the arm, thigh or abdomen.Distribution
Marstacimab‑hncq steady-state apparent volume of distribution is 8.6 L in patients with hemophilia.Elimination
Marstacimab-hncq is cleared via linear and non-linear mechanisms. Marstacimab‑hncq exhibited non‑linear pharmacokinetics due to target-mediated drug disposition (TMDD) which occurs when it forms marstacimab‑hncq/TFPI complex. Once the target becomes saturated, linear pathway (i.e., catabolism) dominates.Based on population pharmacokinetic analysis, 90% of marstacimab is expected to be eliminated by the end of approximately 1 month after the last dose (median time for 50% of drug to be eliminated is approximately 7 to 10 days).
Metabolism
Marstacimab‑hncq is expected to be metabolized into small peptides and amino acids by catabolic pathways in the same manner as endogenous IgG.Specific Populations
No clinically significant differences in pharmacokinetics of marstacimab‑hncq were observed based on race, hemophilia type (A and B), mild renal impairment (eGFR of 60 to 89 mL/min/1.73 m2), and mild hepatic impairment (total bilirubin >1× to ≤1.5× ULN). The effects of geriatric age (>65 years), moderate to severe renal (eGFR <59 mL/min/1.73 m2) and moderate to severe hepatic (Child Pugh class B and C) impairment on marstacimab‑hncq pharmacokinetics are unknown.Body Weight
Body weight was a significant covariate impacting the pharmacokinetics of marstacimab‑hncq. Marstacimab‑hncq exposures over the body weight range of 35 to 120 kg show a trend for increase in exposure with decrease in body weight. However, dose adjustment based on body weight is not required.Pediatric Patients
Marstacimab‑hncq clearance (CL) was 29% lower in adolescents (12 to <18 years of age) compared to adults (18 years and older). No clinically significant difference in adolescent marstacimab‑hncq CL (L/hr/kg) compared to adults was observed after adjusting for body weight.12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and the specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies (ADA) in the studies described below with the incidence of ADA in other studies, including those of marstacimab‑hncq or of other marstacimab products.
During the 12-month active treatment phase in the BASIS study, 23 of the 116 (19.8%) ADA‑evaluable marstacimab‑hncq‑treated patients developed ADAs. Among the 23 patients who tested positive for ADA, 6 patients (26%) developed neutralizing antibodies (NAbs) against marstacimab‑hncq. Subjects who received marstacimab‑hncq and developed anti-marstacimab-hncq antibodies had reduced marstacimab-hncq steady‑state concentrations, geometric mean decrease in the range of 24% to 50%, compared to those who did not develop anti‑marstacimab‑hncq antibodies through the course of the treatment period.
There was no identified clinically significant effect of ADAs, including NAbs, on safety or efficacy of marstacimab‑hncq over the treatment duration of 12 months.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to assess marstacimab‑hncq for the potential for carcinogenicity or mutagenicity. Marstacimab‑hncq did not affect fertility when administered as a repeat dose to male rats at doses up to 1000 mg/kg/dose and an exposure margin of 212-times the exposure at a clinical dose of 300 mg subcutaneous weekly. No effects were observed in male or female reproductive organs in the repeat‑dose toxicity studies of up to 6 months in duration in rats and 3 months in duration in cynomolgus monkeys at doses of 1000 mg/kg/dose and 500 mg/kg/dose and exposure margins at least 201- and 219-times, respectively, the AUC exposure at a clinical dose of 300 mg subcutaneous weekly.
-
14 CLINICAL STUDIES
14.1 Hemophilia A without FVIII Inhibitors or Hemophilia B without FIX Inhibitors
The efficacy of HYMPAVZI was established in 116 adult and pediatric patients (aged 12 years and older and ≥35 kg) with severe hemophilia A without FVIII inhibitors or severe hemophilia B without FIX inhibitors enrolled in the BASIS study (NCT03938792), an open-label, multi-center, two-phase study. Severe hemophilia is defined as factor activity less than 1%. Patients with a history of coronary artery disease, venous or arterial thrombosis or ischemic disease were excluded from the study.
Following screening, patients entered a 6-month observation phase and were enrolled to two cohorts based on the factor replacement treatment they were receiving prior to study entry: on‑demand or routine prophylaxis. Patients who completed the observation phase were to receive 12 months of HYMPAVZI. Of the 116 patients who received HYMPAVZI, 33 patients were in the on‑demand treatment cohort and 83 were in the prophylactic treatment with FVIII or FIX cohort during the observation phase. Patients who completed the 12‑month BASIS study were eligible to enroll in an open-label extension study (NCT05145127).
Patients received an initial 300 mg loading dose of HYMPAVZI followed by maintenance doses of 150 mg of HYMPAVZI once weekly for 12 months. Dose escalation to 300 mg of HYMPAVZI once weekly was permitted after 6 months of treatment in patients weighing ≥50 kg and experiencing ≥2 breakthrough bleeds. Fourteen (12%) underwent dose escalation.
The mean annualized bleeding rates (ABRs) for treated bleeds were 39.86 and 7.90 in the observational phase for the on-demand and prophylaxis cohorts, respectively. All patients in the on-demand cohort had one or more target joints at study entry and 36% had 3 or more target joints at study entry. In the routine prophylaxis cohort, 57% of the patients had one or more target joints at study entry and 16% had 3 or more target joints at study entry.
The efficacy of HYMPAVZI for each cohort was based upon the ABR of treated bleeds during treatment with HYMPAVZI compared to ABR during the observational phase. Other objectives of the study included evaluation of HYMPAVZI prophylaxis on the incidences of spontaneous bleeds, joint bleeds, target joint bleeds and total bleeds.
Among the 116 patients treated with HYMPAVZI in the BASIS study, the mean age was 32 years (range 13 to 66); 19 patients were 12 to <18 years of age and all were male. Fifty-six (56) patients were White, 58 patients were Asian, 1 patient was Black or African American and 1 patient had race information unreported; 12 patients identified as Hispanic or Latino and 104 patients identified as not Hispanic or Latino. The patient population included 91 with hemophilia A and 25 with hemophilia B.
Patients with On-Demand Factor-Based Therapy in Observational Phase
Table 3 shows the efficacy results of HYMPAVZI prophylaxis compared with on-demand factor-based therapy. HYMPAVZI prophylaxis demonstrated superiority over on-demand factor-based therapy in incidences of treated bleeds, spontaneous bleeds, joint bleeds, total bleeds and target joint bleeds.Table 3. Comparison of Annualized Bleeding Rate with HYMPAVZI Prophylaxis Versus On‑Demand Factor-Based Therapy in Patients ≥12 Years of Age without Factor VIII or Factor IX Inhibitors p-value for the null hypothesis that the ratio = 0.5. The estimated mean, ratio, and confidence intervals (CIs) for the ABR come from a negative binomial regression model. Bleed definitions adapted based on ISTH criteria: Treated bleeds = bleeds treated with FVIII or FIX; Total bleeds = bleeds treated and not treated with FVIII or FIX ABR = Annualized Bleeding Rate; CI = Confidence Interval; OD = On-Demand; OP = Observational Phase; ATP = Active Treatment Phase Endpoints in the Order of Testing
HierarchyOn-Demand Factor-Based
Therapy During 6-Month OP
(N = 33)
HYMPAVZI Prophylaxis
During 12-Month ATP
(N = 33)
Treated Bleeds (Primary)
ABR, model-based (95% CI)
39.86 (33.05, 48.07)
3.20 (2.10, 4.88)
Ratio vs. OD (95% CI)
p-value
0.080 (0.057, 0.113)
<0.0001Spontaneous Bleeds, Treated
ABR, model-based (95% CI)
32.63 (25.79, 41.28)
2.45 (1.62, 3.72)
Ratio vs. OD (95% CI)
p-value
0.075 (0.053, 0.107)
<0.0001
Joint Bleeds, Treated
ABR, model-based (95% CI)
34.52 (27.84, 42.79)
2.85 (1.82, 4.46)
Ratio vs. OD (95% CI)
p-value
0.083 (0.057, 0.119)
<0.0001
Total Bleeds, Treated & Untreated
ABR, model-based (95% CI)
49.97 (42.09, 59.32)
7.41 (5.10, 10.75)
Ratio vs. OD (95% CI)
p-value
0.148 (0.111, 0.198)
<0.0001
Target Joint Bleeds, Treated
ABR, model-based (95% CI)
24.38 (18.27, 32.53)
1.84 (1.07, 3.18)
Ratio vs. OD (95% CI)
p-value
0.076 (0.048, 0.119)
<0.0001
Patients with Routine Prophylactic Factor-Based Therapy
Table 4 shows the efficacy results of HYMPAVZI prophylaxis compared with routine prophylactic factor‑based therapy. HYMPAVZI prophylaxis demonstrated non-inferiority to routine prophylactic factor‑based therapy as measured by ABR of treated bleeds as well as incidences of spontaneous bleeds, joint bleeds, target joint bleeds and total bleeds.Table 4. Comparison of Annualized Bleeding Rate with HYMPAVZI Prophylaxis Versus Previous Routine Factor-Based Prophylaxis in Patients ≥12 Years of Age without Factor VIII or Factor IX Inhibitors The protocol specified non-inferiority criterion (upper bound of the 95% CI for the difference) was 2.5 for treated bleeds, spontaneous bleeds, joint bleeds; 1.2 for target joint bleeds; 2.9 for total bleeds. The estimated mean, difference, and confidence intervals (CIs) for the ABR come from negative binomial regression model. Bleed definitions adapted based on ISTH criteria: Treated bleeds = bleeds treated with FVIII or FIX; Total bleeds = bleeds treated and not treated with FVIII or FIX ABR = Annualized Bleeding Rate; CI = Confidence Interval; OP = Observational Phase; ATP = Active Treatment Phase; RP = Routine Prophylaxis Endpoints in the Order of Testing
Hierarchy
Routine Factor-Based
Prophylaxis During
6-Month OP
(N = 83)
HYMPAVZI Prophylaxis
During 12-Month ATP(N = 83)
Treated Bleeds (Primary)
ABR, model-based (95% CI)
7.90 (5.14, 10.66)
5.09 (3.40, 6.78)
Difference vs. RP (95% CI)
-2.81 (-5.42, -0.20)
Spontaneous Bleeds, Treated
ABR, model-based (95% CI)
5.89 (3.57, 8.22)
3.78 (2.25, 5.31)
Difference vs. RP (95% CI)
-2.11 (-4.26, 0.03)
Joint Bleeds, Treated
ABR, model-based (95% CI)
5.69 (3.36, 8.02)
4.13 (2.59, 5.67)
Difference vs. RP (95% CI)
-1.55 (-3.73, 0.62)
Total Bleeds, Treated & Untreated
ABR, model-based (95% CI)
8.90 (6.02, 11.77)
5.98 (4.14, 7.82)
Difference vs. RP (95% CI)
-2.91 (-5.66, -0.17)
Target Joint Bleeds, Treated
ABR, model-based (95% CI)
3.37 (1.60, 5.15)
2.51 (1.26, 3.76)
Difference vs. RP (95% CI)
-0.87 (-2.42, 0.69)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
HYMPAVZI (marstacimab‑hncq) injection is a sterile, preservative-free, clear and colorless to light yellow solution available as a 150 mg/mL single-dose prefilled syringe or pen for subcutaneous administration.
Prefilled Syringe
Each carton (NDC: 0069-1510-01) contains one single-dose prefilled syringe (Type I glass) with a plunger stopper (chlorobutyl elastomer) and a stainless steel 27 gauge, ½ inch staked needle with a rigid needle shield (thermoplastic elastomer).Prefilled Pen
Each carton (NDC: 0069-2151-01) contains one single-dose prefilled pen with needle guard. The syringe inside the pen is made from Type I glass with a plunger stopper (chlorobutyl elastomer) and a stainless steel 27 gauge, ½ inch staked needle with a rigid needle shield (thermoplastic elastomer).HYMPAVZI is not made with natural rubber latex.
16.2 Recommended Storage and Handling
- Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
- If needed, HYMPAVZI may be stored one time at room temperature [up to 86°F (30°C)] in its original carton to protect from light for up to 7 days. Once stored at room temperature, do not return to the refrigerator and discard after 7 days.
- Do not freeze.
- Do not shake.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Ensure that patients and caregivers who will administer HYMPAVZI receive appropriate training and instruction on the proper storage, use and handling of HYMPAVZI from a healthcare professional.
Thromboembolic Events
Inform patients and/or caregivers that HYMPAVZI increases coagulation potential. Discuss the appropriate dosing of concomitant agents such as FVIII or FIX with the patient prior to starting on HYMPAVZI prophylaxis [see Warnings and Precautions (5.1)]. Advise the patient to discontinue HYMPAVZI and seek immediate medical attention if any signs or symptoms of thromboembolism occur.Hypersensitivity
Inform patients and/or caregivers that hypersensitivity reactions such as rash and pruritus are possible. Advise patients to discontinue HYMPAVZI and seek immediate emergency treatment if a severe hypersensitivity reaction occurs [see Warnings and Precautions (5.2)].Pregnancy
Advise female patients of reproductive potential to use effective contraception during treatment with HYMPAVZI and for 2 months after the last dose. Advise patients to report known pregnancies [see Warnings and Precautions (5.3), Use in Specific Populations (8.1, 8.3)].This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
For medical information about HYMPAVZI, please visit www.pfizermedinfo.com or call 1-800-438-1985.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1556-3.0 -
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 09/2025 PATIENT INFORMATION
HYMPAVZI (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
Important information: Before you start using HYMPAVZI, it is very important to talk to your healthcare provider about using factor VIII and factor IX products (products that help blood clot but work in a different way than HYMPAVZI). You may need to use factor VIII or factor IX medicines to treat episodes of breakthrough bleeding during treatment with HYMPAVZI. Carefully follow your healthcare provider’s instructions regarding when to use factor VIII or factor IX medicines and the prescribed dose during your treatment with HYMPAVZI.
What is HYMPAVZI?
HYMPAVZI is a prescription medicine used regularly to prevent or reduce the frequency of bleeding episodes in adults and children 12 years of age and older with hemophilia A without factor VIII inhibitors or hemophilia B without factor IX inhibitors.
It is not known if HYMPAVZI is safe and effective in children younger than 12 years old.
Before using HYMPAVZI, tell your healthcare provider about all of your medical conditions, including if you:
- have a planned surgery. Your healthcare provider may stop treatment with HYMPAVZI before your surgery. Talk to your healthcare provider about when to stop using HYMPAVZI and when to start it again if you have a planned surgery.
- have a severe short-term (acute) illness such as an infection or injury.
- have been told that you have a risk for blood clots.
- are pregnant or plan to become pregnant. HYMPAVZI may harm your unborn baby.
-
Females who are able to become pregnant:
- o Your healthcare provider will do a pregnancy test before you start your treatment with HYMPAVZI.
- o You should use effective birth control (contraception) during treatment with HYMPAVZI and for 2 months after the last dose of HYMPAVZI.
- o Tell your healthcare provider right away if you become pregnant or think that you may be pregnant during treatment with HYMPAVZI.
- are breastfeeding or plan to breastfeed. It is not known if HYMPAVZI passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over‑the‑counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use HYMPAVZI?
See the detailed “Instructions for Use” that comes with your HYMPAVZI for information on how to inject a dose of HYMPAVZI, and how to properly throw away (dispose of) used HYMPAVZI prefilled syringe or HYMPAVZI prefilled pen.
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- Your healthcare provider will provide instructions for stopping (discontinuing) your current treatment when switching from factor- or non-factor-based medicines to HYMPAVZI.
- HYMPAVZI is given as an injection under your skin (subcutaneous injection) by you or your caregiver.
- Your healthcare provider should show you or your caregiver how to inject HYMPAVZI before you inject yourself for the first time.
- Do not attempt to inject yourself or another person unless you have been taught how to do so by a healthcare provider.
- Your first dose (loading dose) of HYMPAVZI is 300 mg (two 150 mg injections). Then you will inject a weekly (maintenance) dose as prescribed by your healthcare provider.
- Inject HYMPAVZI 1 time a week on the same day each week. Inject HYMPAVZI at any time of day.
- Your healthcare provider will provide information on the treatment of breakthrough bleeding during your treatment with HYMPAVZI. Do not use HYMPAVZI to treat breakthrough bleeding.
-
If you miss a maintenance dose of 150 mg of HYMPAVZI:
- o Give the dose as soon as possible before the next scheduled dose, and then continue with your usual 150 mg weekly dosing schedule. You may inject your next dose at your regularly scheduled time or continue dosing based on the new day you injected your missed dose. In case you are not sure when to inject HYMPAVZI, call your healthcare provider.
- o If more than 13 days have passed since your last dose was given, call your healthcare provider right away for dosing instructions.
-
If you miss 1 or more doses of maintenance dose of 300 mg of HYMPAVZI:
- o Give a dose as soon as possible, and then continue with your 300 mg weekly dosing schedule. You may inject your next dose at your regularly scheduled time or continue dosing based on the new day you injected your missed dose. In case you are not sure when to inject HYMPAVZI, call your healthcare provider.
What are the possible side effects of HYMPAVZI?
HYMPAVZI may cause serious side effects, including:
- blood clots (thromboembolic events). HYMPAVZI may increase the risk for your blood to clot in blood vessels in your arm, leg, lung, or head and can be life-threatening. Blood clots have happened in people using HYMPAVZI. You may have an increased risk of blood clots if you have certain risk factors. Stop using HYMPAVZI and get medical help right away if you develop any of these signs or symptoms of blood clots:
- o swelling or pain in arms or legs
- o redness or discoloration in your arms or legs
- o shortness of breath
- o pain in chest or upper back
- o fast heart rate
- o cough up blood
- o feel faint
- o headache
- o numbness in your face
- o eye pain or swelling
- o trouble seeing
- allergic reactions. HYMPAVZI may cause allergic reactions, including rash and itching. Stop using HYMPAVZI and get medical help right away if you develop any of the following symptoms of a severe allergic reaction:
- o swelling of your face, lips, mouth, or tongue
- o trouble breathing
- o wheezing
- o dizziness or fainting
- o fast heartbeat or pounding in your chest
- o sweating
The most common side effects of HYMPAVZI include:
- swelling, hardening, redness, bruising, and pain at injection site
- headache
- itching
These are not all of the possible side effects of HYMPAVZI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store HYMPAVZI?
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- Store HYMPAVZI in the original carton to protect from light.
- If needed, HYMPAVZI may be stored one time at room temperature up to 86°F (30°C) in its original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- Do not freeze HYMPAVZI.
- Do not shake HYMPAVZI.
Keep HYMPAVZI and all medicines out of the reach of children.
General information about the safe and effective use of HYMPAVZI.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use HYMPAVZI for a condition for which it was not prescribed. Do not give HYMPAVZI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about HYMPAVZI that is written for health professionals.
What are the ingredients in HYMPAVZI?
Active ingredient: marstacimab‑hncq
Inactive ingredients: edetate disodium, histidine, L-histidine monohydrochloride, polysorbate 80, sucrose, and water for injection
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.

US License No. 2001
Distributed by: Pfizer Labs, Division of Pfizer Inc., New York, NY 10001
LAB-1575-2.0
For more information, go to website www.HYMPAVZI.com or call 1-800-438-1985. -
INSTRUCTIONS FOR USE
HYMPAVZI™ (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
single-dose prefilled syringe
This Instructions for Use contains information on how to inject HYMPAVZI.
Read this Instructions for Use carefully before using HYMPAVZI Prefilled Syringe and each time you get a refill prescription as there may be new information.
Your healthcare provider should show you or your caregiver how to prepare and inject a dose of HYMPAVZI the right way before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject HYMPAVZI.
Important Information You Need to Know Before Injecting HYMPAVZI
- Each HYMPAVZI Prefilled Syringe is a Single-Dose Prefilled Syringe (called “Syringe” in this Instructions for Use). The HYMPAVZI Prefilled Syringe contains 150 mg of HYMPAVZI for injection under the skin (subcutaneously).
- Do not inject HYMPAVZI into a vein.
- To help you remember when to inject HYMPAVZI, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject HYMPAVZI, or call the helpline at 1‑888‑496‑7289 (1‑888 HYMPAV‑Z).
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- HYMPAVZI is not made with natural rubber latex.
Storing HYMPAVZI
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- Store HYMPAVZI in the original carton to protect from light.
- If needed, HYMPAVZI may be stored one time at room temperature, up to 86°F (30°C) in the original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- Do not freeze HYMPAVZI.
- Do not shake HYMPAVZI.
- Do not use past the expiration date (Exp) printed on the HYMPAVZI Prefilled Syringe.
- Keep HYMPAVZI and all medicines out of the reach of children.
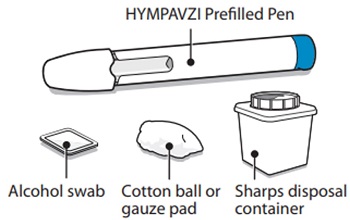
Supplies Needed For HYMPAVZI InjectionGather the following supplies on a clean flat surface:
Included in the carton:
- 1 HYMPAVZI Prefilled Syringe
Not included in the carton:
- 1 alcohol swab
- 1 cotton ball or gauze pad
- 1 FDA-cleared sharps disposal container for Syringe disposal (see “Step 11 – Disposal of Syringe” and “Safe Syringe Disposal” information section)
HYMPAVZI Prefilled SyringeAlways hold HYMPAVZI Prefilled Syringe by the barrel to prevent damage.
Preparing to Inject HYMPAVZIStep 1 – Getting Ready
- Remove the Syringe from its carton and keep out of direct sunlight.
- Make sure the name HYMPAVZI appears on the carton and Syringe label.
- Check the Syringe for any visible damage such as cracks or leaks.
- Wash and dry your hands.
- Do not remove the needle cover until you are ready to inject.
- Throw away (dispose of) the Syringe if it is damaged, or if the Syringe or the carton containing the Syringe has been dropped.
-
Do not use the Syringe if:
- o it has been stored in direct light. Exposure to room light during dose preparation and injection is acceptable.
- o it has been frozen or thawed or it has been out of the refrigerator for more than 7 days.
Note: For a more comfortable injection, allow the Syringe to warm up to room temperature [up to 86°F (30°C)] in the carton away from direct sunlight for about 15 to 30 minutes.
Do not use any other methods to warm up the Syringe, such as warming the Syringe in a microwave or hot water.
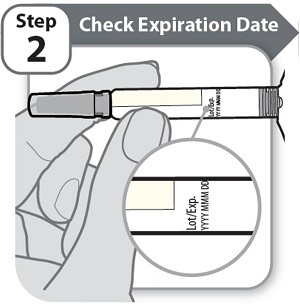
Step 2 – Check Expiration Date- Check the expiration date (Exp) printed on the Syringe label.
- Do not use if the expiration date (Exp) has passed.
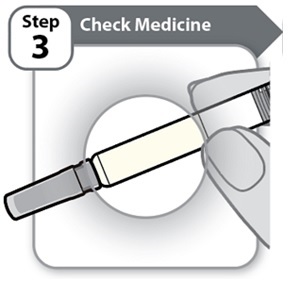
Step 3 – Check Medicine- Gently tilt the Syringe back and forth.
-
Look carefully at the medicine in the Syringe.
- o The medicine should be clear and colorless to light yellow.
- o Do not use the Syringe if the medicine is cloudy, dark yellow, or contains flakes or particles.
Note: It is normal to see air bubbles in the Syringe.
If you have any questions about the medicine, contact your healthcare provider.
Step 4 – Choose and Clean Your Injection Site- Choose an injection site on your stomach area (abdomen) or front of thigh unless a different site has been suggested by your healthcare provider. HYMPAVZI may also be injected into the back of your upper arms by a healthcare provider or caregiver only. Keep at least 2 inches away from your belly button.
- Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI and away from any other medicine given under your skin. You may use the same area of your body but be sure to choose a different injection site in that area.
- Clean the injection site with soap and water, or an alcohol swab.
- Allow the site to air dry. Do not touch, fan, or blow on the cleaned injection site.
- Do not inject HYMPAVZI into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- Do not inject HYMPAVZI into a vein.
- Do not inject HYMPAVZI through your clothes.
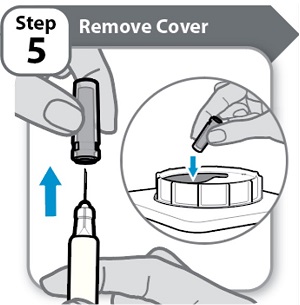
Step 5 – Remove Cover- Hold the Syringe by the barrel.
- Pull the needle cover straight off carefully.
- Put the needle cover into an FDA-cleared sharps disposal container right away. You will not need it again.
- Do not touch the needle or let it touch any surfaces.
Note: It is normal to see a few drops of medicine at the needle tip.
Caution: Handle the Syringe with care to avoid an accidental needle injury.
Injecting HYMPAVZIStep 6 – Insert Needle
- Pinch your cleaned skin between your thumb and fingers to create a firm surface.
- Fully insert the needle into your skin at a 45° angle, as shown. Do not hold or push on the plunger while inserting the needle.
Keep your skin pinched throughout the injection.
Caution: If you change your mind where to inject after inserting the needle into your skin, you will need to throw away (dispose of) the Syringe and get a new HYMPAVZI Prefilled Syringe.
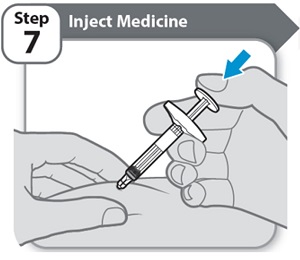
Step 7 – Inject Medicine- Slowly inject all of HYMPAVZI by gently pushing the plunger rod all the way down, until the barrel is empty.
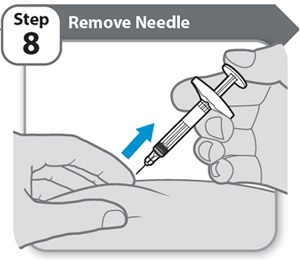
Step 8 – Remove Needle- Pull the needle and Syringe out of your skin at the same angle as inserted.
Note: If you see a small drop of medicine on your skin, wait a little longer before removing the needle when you give your next injection.
Step 9 – Check Syringe- Check the Syringe to make sure the gray plunger stopper is in the position shown.
If the gray plunger stopper is not in the position shown, this means you have not received a full dose. Call your healthcare provider for help.
Never re-insert the needle.
Do not inject another dose.
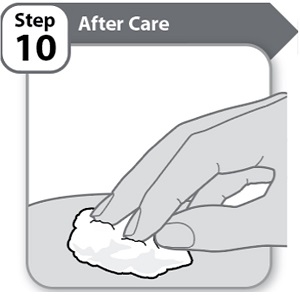
Step 10 – After Care- Press lightly on the injection site for a few seconds with a clean cotton ball or gauze pad if you see a drop of blood.
- Do not rub the area.
Note: If bleeding does not stop, contact your healthcare provider.
Note: If your prescribed dose requires 2 injections of HYMPAVZI, repeat steps 1-10. Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI. You may use the same area of your body but be sure to choose a different injection site in that area.
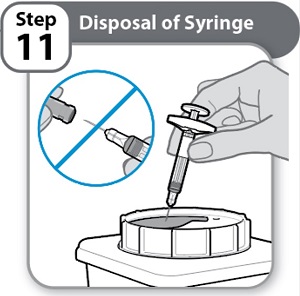
- Put the used Syringe in an FDA-cleared sharps disposal container right away after use.
Never re-cap the needle.
- Do not throw away (dispose of) Syringes in the household trash.
Note: If you do not have an FDA-cleared sharps disposal container, please see the “Safe Syringe Disposal” information section.
- Always throw away (dispose of) Syringes in a sharps disposal container. Do not dispose of Syringes in the household trash.
-
If you do not have an FDA-cleared sharps disposal container, you may use a household container that:
- o is made of heavy-duty plastic,
- o can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o is upright and stable during use,
- o is leak-resistant, and
- o is properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away (dispose of) your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about safe sharps disposal for the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1576-2.0For more information, go to www.pfizer.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 09/2025
-
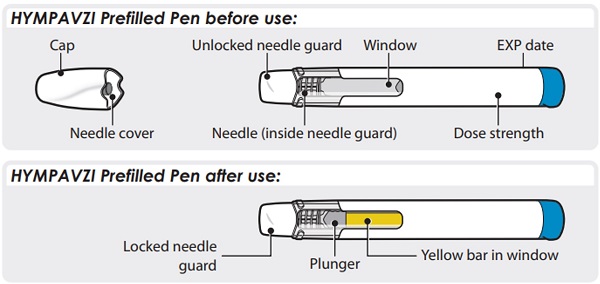
INSTRUCTIONS FOR USE
HYMPAVZI™ (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
single-dose prefilled penThis Instructions for Use contains information on how to inject HYMPAVZI.
Read this Instructions for Use carefully before using HYMPAVZI Prefilled Pen and each time you get a refill prescription as there may be new information.
Your healthcare provider should show you or your caregiver how to prepare and inject a dose of HYMPAVZI the right way before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject HYMPAVZI.
Important Information You Need to Know Before Injecting HYMPAVZI
- Each HYMPAVZI Prefilled Pen is a Single-Dose Prefilled Pen (called “Pen” in this Instructions for Use). The HYMPAVZI Prefilled Pen contains 150 mg of HYMPAVZI for injection under the skin (subcutaneously).
- Do not inject HYMPAVZI into a vein.
- To help you remember when to inject HYMPAVZI, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject HYMPAVZI, or call the helpline at 1‑888‑496‑7289 (1‑888 HYMPAV‑Z).
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- HYMPAVZI is not made with natural rubber latex.
Storing HYMPAVZI
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- Store HYMPAVZI in the original carton to protect from light.
- If needed, HYMPAVZI may be stored one time at room temperature, up to 86°F (30°C) in the original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- Do not freeze HYMPAVZI.
- Do not shake HYMPAVZI.
- Do not use past the expiration date (EXP) printed on the HYMPAVZI Prefilled Pen.
- Keep HYMPAVZI and all medicines out of the reach of children.
Supplies Needed For HYMPAVZI InjectionGather the following supplies on a clean flat surface:
Included in the carton:
- 1 HYMPAVZI Prefilled Pen
Not included in the carton:
- 1 alcohol swab
- 1 cotton ball or gauze pad
- 1 FDA-cleared sharps disposal container for Pen disposal (see “Step 10 – Disposal of Pen” and “Safe Pen Disposal” information section)
HYMPAVZI Prefilled Pen
Preparing to Inject HYMPAVZIStep 1 – Getting Ready
- Remove the Pen from its carton and keep out of direct sunlight.
- Make sure the name HYMPAVZI appears on the carton and Pen label.
- Check the Pen for any visible damage such as cracks or leaks.
- Wash and dry your hands.
- Do not remove the cap until you are ready to inject.
- Throw away (dispose of) the Pen if it is damaged, or if the Pen or the carton containing the Pen has been dropped.
-
Do not use the Pen if:
- o it has been stored in direct light. Exposure to room light during dose preparation and injection is acceptable.
- o it has been frozen or thawed or it has been out of the refrigerator for more than 7 days.
Note: For a more comfortable injection, allow the Pen to warm up to room temperature [up to 86°F (30°C)] in the carton away from direct sunlight for about 15 to 30 minutes.
Do not use any other methods to warm up the Pen, such as warming the Pen in a microwave or hot water.
Step 2 – Check Expiration Date- Check the expiration date (EXP) printed on the Pen label.
- Do not use if the expiration date (EXP) has passed.
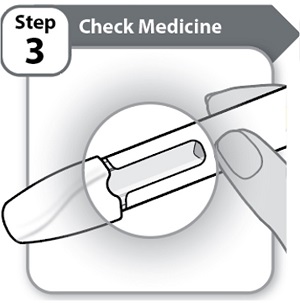
Step 3 – Check Medicine-
Look carefully at the medicine through the window on the Pen.
- o The medicine should be clear and colorless to light yellow.
- o Do not use the Pen if the medicine is cloudy, dark yellow, or contains flakes or particles.
Note: It is normal to see air bubbles in the window.
If you have any questions about the medicine, contact your healthcare provider.
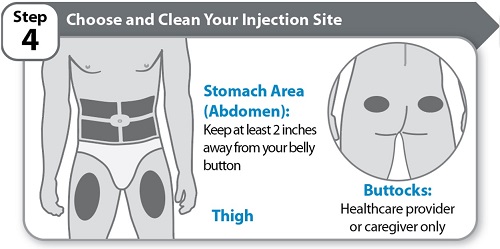
Step 4 – Choose and Clean Your Injection Site- Choose an injection site on your stomach area (abdomen) or front of thigh unless a different site has been suggested by your healthcare provider. HYMPAVZI may also be injected into your buttocks by a healthcare provider or caregiver only. Keep at least 2 inches away from your belly button.
- Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI and away from any other medicine given under your skin. You may use the same area of your body but be sure to choose a different injection site in that area.
- Clean the injection site with soap and water, or an alcohol swab.
- Allow the site to air dry. Do not touch, fan, or blow on the cleaned injection site.
- Do not inject HYMPAVZI into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- Do not inject HYMPAVZI into a vein.
- Do not inject HYMPAVZI through your clothes.
Step 5 – Twist Off Cap- Twist and pull off the cap.
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
Note:
-
- o It is normal to see a few drops of medicine at the needle tip.
- o The needle cover will stay inside the cap after cap removal.
Caution: Handle the Pen with care as it contains a needle.
Do not put or press your hand over the needle guard. Doing so may cause a needle injury.
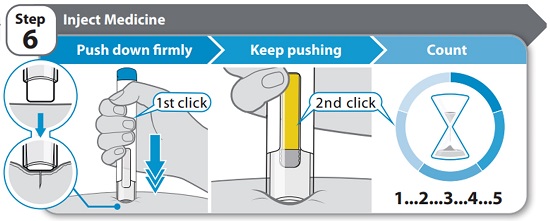
Injecting HYMPAVZIStep 6 – Inject Medicine
- Hold the Pen straight (at 90° angle) against your cleaned skin so you can see the window.
- Push the Pen down firmly straight against your skin and keep pushing until the injection is complete. You will hear the 1st click when the injection starts.
- Keep pushing the Pen firmly against your skin while the yellow bar moves across the window. You will hear a 2nd click when the injection is almost complete.
- Count slowly to 5 after you hear the 2nd click to make sure you get a full dose.
Do not remove the Pen from your skin until you have counted slowly to 5 after you hear the 2nd click and until the yellow marker completely fills the window (see “Step 7 – Remove Pen”).
Note: The needle goes into your skin as you push the Pen down. Your healthcare provider may suggest gently pinching your skin while you inject.
Note: If you do not hear a click when pushing the Pen against your skin, try pushing down harder. If you still cannot start the injection, get a new HYMPAVZI Prefilled Pen.
Caution: If you change your mind where to inject after inserting the needle into your skin, you will need to throw away (dispose of) the Pen and get a new HYMPAVZI Prefilled Pen.
-
Remove the Pen from your skin.
- o If you see a small drop of medicine on your skin, wait a little longer before removing the Pen when you give your next injection.
Note: After you remove the Pen from your skin, the needle guard will automatically cover the needle and lock in place.
The Pen cannot be reused.
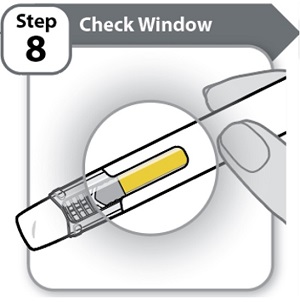
Step 8 – Check Window- Check the window to make sure all the medicine has been injected.
If the yellow bar is not in the position shown, this means you have not received a full dose. Call your healthcare provider for help.
Do not inject another dose.
Step 9 – After Care- Press lightly on the injection site for a few seconds with a clean cotton ball or gauze pad if you see a drop of blood.
- Do not rub the area.
Note: If bleeding does not stop, contact your healthcare provider.
Note: If your prescribed dose requires 2 injections of HYMPAVZI, repeat steps 1-9. Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI. You may use the same area of your body but be sure to choose a different injection site in that area.
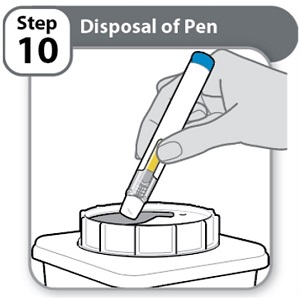
- Put the used Pen in an FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) Pens in the household trash.
Note: If you do not have an FDA-cleared sharps disposal container, please see the “Safe Pen Disposal” information section.
- Always throw away (dispose of) Pens in a sharps disposal container. Do not dispose of Pens in the household trash.
-
If you do not have an FDA-cleared sharps disposal container, you may use a household container that:
- o is made of heavy-duty plastic,
- o can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o is upright and stable during use,
- o is leak-resistant, and
- o is properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away (dispose of) your sharps disposal container. There may be state or local laws about how you should throw away used needles and Pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal for the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1577-2.0
For more information, go to www.pfizer.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 09/2025
-
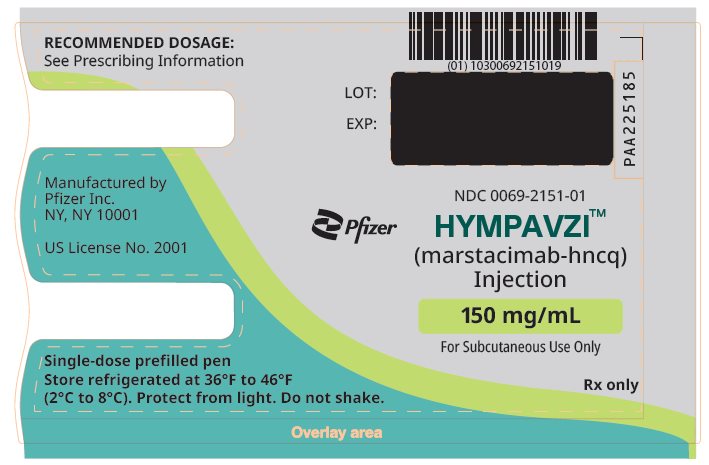
PRINCIPAL DISPLAY PANEL – 150 mg/mL Prefilled Pen
NDC: 0069-2151-01
Pfizer
HYMPAVZITM
(marstacimab-hncq)
Injection150 mg/mL
For Subcutaneous Use Only
Rx only
-
PRINCIPAL DISPLAY PANEL – 150 mg/mL Prefilled Pen Carton
NDC: 0069-2151-01
1 single-dose prefilled pen
Pfizer
HYMPAVZI TM
(marstacimab-hncq)
Injection150 mg/mL
For Subcutaneous Use Only
READ ENCLOSED INSTRUCTIONS BEFORE USEThis carton contains:
1 Prefilled Pen
1 Instructions for Use
1 Prescribing InformationKeep out of reach of children.
Rx only
-
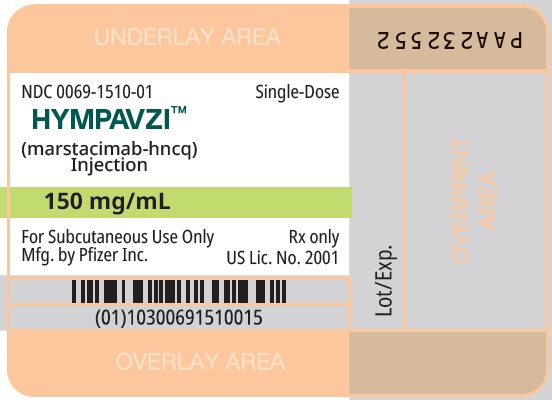
PRINCIPAL DISPLAY PANEL - 150 mg/mL Prefilled Syringe
NDC: 0069-1510-01
Single-Dose
HYMPAVZITM
(marstacimab-hncq)
Injection150 mg/mL
For Subcutaneous Use Only
Rx only
Mfg. by Pfizer Inc.
US Lic. No. 2001
-
PRINCIPAL DISPLAY PANEL - 150 mg/mL Prefilled Syringe Carton
NDC: 0069-1510-01
1 single-dose prefilled syringe
Pfizer
HYMPAVZI TM
(marstacimab-hncq)
Injection150 mg/mL
For Subcutaneous Use Only
READ ENCLOSED INSTRUCTIONS BEFORE USEThis carton contains:
1 Prefilled Syringe
1 Instructions for Use
1 Prescribing InformationKeep out of reach of children.
Rx only
-
INGREDIENTS AND APPEARANCE
HYMPAVZI
marstacimab-hncq injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0069-2151 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MARSTACIMAB (UNII: 0UB3OA67O7) (MARSTACIMAB - UNII:0UB3OA67O7) MARSTACIMAB 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 1.12 mg in 1 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 2.67 mg in 1 mL SUCROSE (UNII: C151H8M554) 85 mg in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.05 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.2 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-2151-01 1 in 1 CARTON 11/05/2024 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761369 11/05/2024 HYMPAVZI
marstacimab-hncq injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0069-1510 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MARSTACIMAB (UNII: 0UB3OA67O7) (MARSTACIMAB - UNII:0UB3OA67O7) MARSTACIMAB 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 1.12 mg in 1 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 2.67 mg in 1 mL SUCROSE (UNII: C151H8M554) 85 mg in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.05 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.2 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-1510-01 1 in 1 CARTON 10/16/2025 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761369 10/16/2025 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Establishment Name Address ID/FEI Business Operations Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC 174350868 API MANUFACTURE(0069-2151, 0069-1510) , ANALYSIS(0069-2151, 0069-1510) Establishment Name Address ID/FEI Business Operations Pfizer Manufacturing Belgium NV 370156507 MANUFACTURE(0069-2151, 0069-1510) , ANALYSIS(0069-2151, 0069-1510) , PACK(0069-2151, 0069-1510) , LABEL(0069-2151, 0069-1510) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals Unlimited Company 985586408 ANALYSIS(0069-2151, 0069-1510)
Trademark Results [HYMPAVZI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HYMPAVZI 90863217 not registered Live/Pending |
Pfizer Inc. 2021-08-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.