Carbon Dioxide by MESSER GAS PUERTO RICO, INC / Messer Gas Puerto Rico Inc CARBON DIOXIDE gas
Carbon Dioxide by
Drug Labeling and Warnings
Carbon Dioxide by is a Prescription medication manufactured, distributed, or labeled by MESSER GAS PUERTO RICO, INC, Messer Gas Puerto Rico Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
PRINCIPAL DISPLAY PANEL
Bulk Carbon Dioxide USP Certificate of Analysis
This is to certify that the carbon dioxide tested at MESSER PLANT on 10/27/2025, meets the United States Pharmacopoeia (USP) specifications, the requirements of Federal Specifications BB-C-101 Grade A and QVL E of CGA G6.2. This product was manufactured at a facility registered with the Federal Food and Drug Administration and is approved for prescription drug use. This product is not guaranteed to meet any other specification or intended for pesticide use. NDA 205764 NADA 141362
Furthermore, batch number 5784-A-102725-01, and lot number TCO2002-102725-13, which was loaded on 10/27/2025 and produced at MESSER PLANT comprises this shipment and was analyzed as shown below.
Analytical results are generated using USP monograph or validated equivalent methods.
FOR MEDICAL USE
Requirement Analytical Method Limit of Detection¹ Specification Limit Result
Carbon Dioxide Assay (CO₂) Speed of Sound 99.99% 99.0% 99.99%
Nitrogen Oxide (NO) IMR-MS 0.05 ppm 2.5 ppm ND
Nitrogen Dioxide (NO₂) IMR-MS 0.1 ppm 2.5 ppm ND
Ammonia (NH₃) IMR-MS 0.03 ppm 25 ppm ND
Sulfur Dioxide (SO₂) IMR-MS 0.03 ppm 5 ppm ND
Hydrogen Sulfide (H₂S) IMR-MS 0.03 ppm 1 ppm ND
Carbon Monoxide (CO) FTIR 0.05 ppm 10 ppm ND
Moisture (H₂O) Quartz Crystal 0.5 ppm 200 ppm 0.77 ppm
Notes:
1. Positive Carbon Dioxide Identification is achieved when the Assay results demonstrate meeting the specification.
2. The limit of detection (LOO) for each test method is the threshold value for results to be reported with certainty in the accuracy of results. A test result outside the detection limit for the test method will be reported as ND.
3. ND = None Detected
4. ppm = parts per million by volume, ppb = parts per billion by volume, 1 ppm = 1,000 ppb
Product analyzed using Messer Ultra Guard Suite
Certified By:
On:
(Signature) (Date)
John Doe
(Print Name & Title)
On behalf of Messer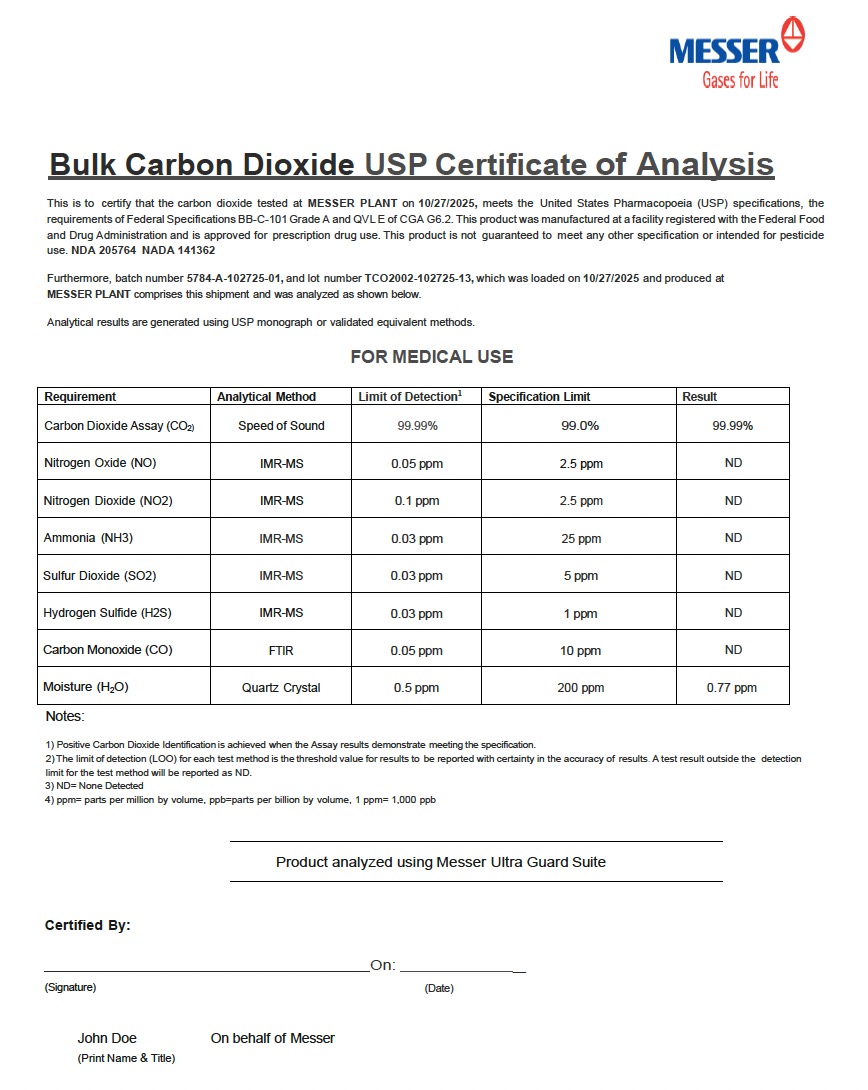
-
INGREDIENTS AND APPEARANCE
CARBON DIOXIDE
carbon dioxide gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52374-103 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBON DIOXIDE (UNII: 142M471B3J) (CARBON DIOXIDE - UNII:142M471B3J) CARBON DIOXIDE 990 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52374-103-01 21000 L in 1 CONTAINER; Type 0: Not a Combination Product 01/01/1965 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205815 01/01/1965 Labeler - MESSER GAS PUERTO RICO, INC (090044413) Registrant - MESSER GAS PUERTO RICO, INC (090044413) Establishment Name Address ID/FEI Business Operations Messer Gas Puerto Rico Inc 049495666 manufacture(52374-103)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.