LASTACAFT- alcaftadine solution/ drops
LASTACAFT by
Drug Labeling and Warnings
LASTACAFT by is a Otc medication manufactured, distributed, or labeled by Allergan, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use
■ if solution changes color or becomes cloudy
■ if you are sensitive to any ingredient in this product
■ to treat contact lens related irritation
When using this product
■ do not touch tip of container to any surface to avoid contamination
■ remove contact lenses before use
■ wait at least 10 minutes before reinserting contact lenses after use
■ do not wear a contact lens if your eye is red
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
NDC: 0023-4291-10
ORIGINAL
PRESCRIPTION STRENGTH
LASTACAFT®
Alcaftadine ophthalmic solution 0.25%
Antihistamine Eye Drops
ONCE DAILY RELIEF
Eye Allergy Itch Relief
WORKS IN MINUTES
RELIEF FROM ALLERGENS:
Pet Dander Pollen Grass Ragweed
STERILE
120 DAY SUPPLY
Two 5 mL bottles (0.17 fl oz each)
-
PRINCIPAL DISPLAY PANEL
NDC: 0023-4291-05
ORIGINAL
PRESCRIPTION STRENGTH
LASTACAFT®
Alcaftadine ophthalmic solution 0.25%
Antihistamine Eye Drops
ONCE DAILY RELIEF
Eye Allergy Itch Relief
WORKS IN MINUTES
RELIEF FROM ALLERGENS:
Pet Dander Pollen Grass Ragweed
STERILE
60 DAY SUPPLY
0.17 fl oz (5 mL)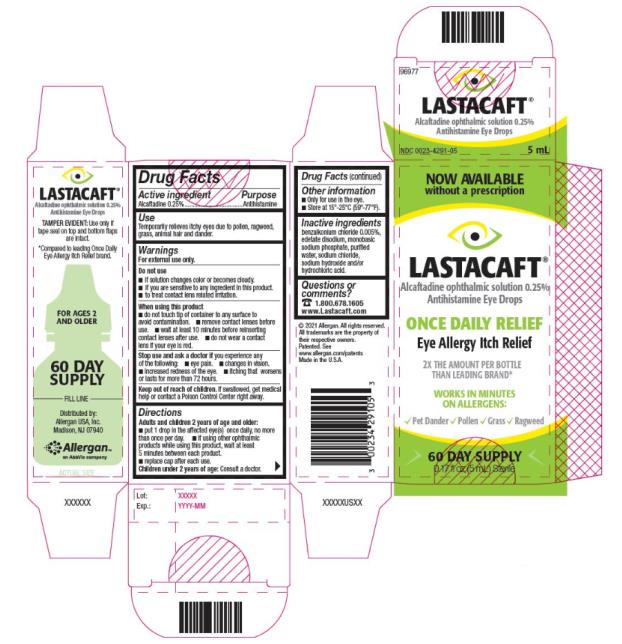
-
INGREDIENTS AND APPEARANCE
LASTACAFT
alcaftadine solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0023-4291 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength alcaftadine (UNII: 7Z8O94ECSX) (alcaftadine - UNII:7Z8O94ECSX) alcaftadine 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) edetate disodium (UNII: 7FLD91C86K) sodium phosphate, monobasic (UNII: 3980JIH2SW) water (UNII: 059QF0KO0R) sodium chloride (UNII: 451W47IQ8X) sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-4291-05 1 in 1 CARTON 12/01/2021 1 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 0023-4291-10 2 in 1 CARTON 12/01/2021 2 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC: 0023-4291-01 1 in 1 CARTON 12/01/2021 3 1 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC: 0023-4291-06 1 in 1 CARTON 12/01/2021 4 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 5 NDC: 0023-4291-07 2 in 1 CARTON 12/01/2021 5 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022134 12/01/2021 Labeler - Allergan, Inc. (144796497)
Trademark Results [LASTACAFT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LASTACAFT 85168259 4071760 Live/Registered |
Allergan, Inc. 2010-11-03 |
 LASTACAFT 85088422 4003795 Live/Registered |
Allergan, Inc. 2010-07-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
