FOMEPIZOLE injection, solution
fomepizole by
Drug Labeling and Warnings
fomepizole by is a Prescription medication manufactured, distributed, or labeled by Sandoz Inc. , Emcure Pharmaceuticals Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Fomepizole Injection is a competitive inhibitor of alcohol dehydrogenase. The chemical name of fomepizole is 4-methylpyrazole. It has the molecular formula C4H6N2 and a molecular weight of 82.1. The structural formula is:
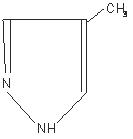
It is a clear to yellow liquid at room temperature. Its melting point is 25°C (77°F) and it may present in a solid form at room temperature. Fomepizole is soluble in water and very soluble in ethanol, diethyl ether, and chloroform. Each vial contains 1.5 mL (1 g/mL) of fomepizole. -
CLINICAL PHARMACOLOGY
Mechanism of Action : Fomepizole is a competitive inhibitor of alcohol dehydrogenase. Alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde. Alcohol dehydrogenase also catalyzes the initial steps in the metabolism of ethylene glycol and methanol to their toxic metabolites.
Ethylene glycol, the main component of most antifreezes and coolants, is metabolized to glycoaldehyde, which undergoes subsequent sequential oxidations to yield glycolate, glyoxylate, and oxalate. Glycolate and oxalate are the metabolic byproducts primarily responsible for the metabolic acidosis and renal damage seen in ethylene glycol toxicosis. The lethal dose of ethylene glycol in humans is approximately 1.4 mL/kg.
Methanol, the main component of windshield wiper fluid, is slowly metabolized via alcohol dehydrogenase to formaldehyde with subsequent oxidation via formaldehyde dehydrogenase to yield formic acid. Formic acid is primarily responsible for the metabolic acidosis and visual disturbances (e.g., decreased visual acuity and potential blindness) associated with methanol poisoning. A lethal dose of methanol in humans is approximately 1 to 2 mL/kg.
Fomepizole has been shown in vitro to block alcohol dehydrogenase enzyme activity in dog, monkey, and human liver. The concentration of fomepizole at which alcohol dehydrogenase is inhibited by 50% in vitro is approximately 0.1 µmol/L.
In a study of dogs given a lethal dose of ethylene glycol, three animals each were administered fomepizole, ethanol, or left untreated (control group). The three animals in the untreated group became progressively obtunded, moribund, and died. At necropsy, all three dogs had severe renal tubular damage. Fomepizole or ethanol, given 3 hours after ethylene glycol ingestion, attenuated the metabolic acidosis and prevented the renal tubular damage associated with ethylene glycol intoxication.
Several studies have demonstrated that fomepizole plasma concentrations of approximately 10 µmol/L (0.82 mg/L) in monkeys are sufficient to inhibit methanol metabolism to formate, which is also mediated by alcohol dehydrogenase. Based on these results, concentrations of fomepizole in humans in the range of 100 to 300 µmol/L (8.6 to 24.6 mg/L) have been targeted to assure adequate plasma concentrations for the effective inhibition of alcohol dehydrogenase.
In healthy volunteers, oral doses of fomepizole (10 to 20 mg/kg) significantly reduced the rate of elimination of moderate doses of ethanol, which is also metabolized through the action of alcohol dehydrogenase (see PRECAUTIONS, Drug Interactions).
Pharmacokinetics : The plasma half-life of fomepizole varies with dose, even in patients with normal renal function, and has not been calculated.
Distribution : After intravenous infusion, fomepizole rapidly distributes to total body water. The volume of distribution is between 0.6 L/kg and 1.02 L/kg.
Metabolism : In healthy volunteers, only 1 to 3.5% of the administered dose of fomepizole (7 to 20 mg/kg oral and IV) was excreted unchanged in the urine, indicating that metabolism is the major route of elimination. In humans, the primary metabolite of fomepizole is 4-carboxypyrazole (approximately 80 to 85% of administered dose), which is excreted in the urine. Other metabolites of fomepizole observed in the urine are 4-hydroxymethylpyrazole and the N-glucuronide conjugates of 4-carboxypyrazole and 4-hydroxymethylpyrazole.
Excretion : The elimination of fomepizole is best characterized by Michaelis-Menten kinetics after acute doses, with saturable elimination occurring at therapeutic blood concentrations [100 to 300 µmol/L, 8.2 to 24.6 mg/L].
With multiple doses, fomepizole rapidly induces its own metabolism via the cytochrome P450 mixed-function oxidase system, which produces a significant increase in the elimination rate after about 30 to 40 hours. After enzyme induction, elimination follows first-order kinetics.
Special Populations :
Geriatric : Fomepizole Injection has not been studied sufficiently to determine whether the pharmacokinetics differ for a geriatric population.
Pediatric : Fomepizole has not been studied sufficiently to determine whether the pharmacokinetics differ for a pediatric population.
Gender : Fomepizole has not been studied sufficiently to determine whether the pharmacokinetics differ between the genders.
Renal Insufficiency : The metabolites of fomepizole are excreted renally. Definitive pharmacokinetic studies have not been done to assess pharmacokinetics in patients with renal impairment.
Hepatic Insufficiency : Fomepizole is metabolized through the liver, but no definitive pharmacokinetic studies have been done in subjects with hepatic disease.
Clinical Studies:
The efficacy of fomepizole in the treatment of ethylene glycol and methanol intoxication was studied in two prospective, U.S. clinical trials without concomitant control groups. Fourteen of 16 patients in the ethylene glycol trial and 7 of 11 patients in the methanol trial underwent hemodialysis because of severe intoxication (see DOSAGE AND ADMINISTRATION). All patients received fomepizole shortly after admission.
The results of these two studies provide evidence that fomepizole blocks ethylene glycol and methanol metabolism mediated by alcohol dehydrogenase in the clinical setting. In both studies, plasma concentrations of toxic metabolites of ethylene glycol and methanol failed to rise in the initial phases of treatment. The relationship to fomepizole therapy, however, was confounded by hemodialysis and significant blood ethanol concentrations in many of the patients. Nevertheless, in the post-dialysis period(s), when ethanol concentrations were insignificant and the concentrations of ethylene glycol or methanol were > 20 mg/dL, the administration of fomepizole alone blocked any rise in glycolate or formate concentrations, respectively.
In a separate French trial, 5 patients presented with ethylene glycol concentrations ranging from 46.5 to 345 mg/dL, insignificant ethanol blood concentrations, and normal renal function. These patients were treated with fomepizole alone without hemodialysis, and none developed signs of renal injury. -
INDICATIONS & USAGE
Fomepizole is indicated as an antidote for ethylene glycol (such as antifreeze) or methanol poisoning, or for use in suspected ethylene glycol or methanol ingestion, either alone or in combination with hemodialysis (see DOSAGE AND ADMINISTRATION). - CONTRAINDICATIONS
-
PRECAUTIONS
General
Fomepizole should not be given undiluted or by bolus injection. Venous irritation and phlebosclerosis were noted in two of six normal volunteers given bolus injections (over 5 minutes) of fomepizole at a concentration of 25 mg/mL.
Minor allergic reactions (mild rash, eosinophilia) have been reported in a few patients receiving fomepizole (see ADVERSE REACTIONS). Therefore, patients should be monitored for signs of allergic reactions.Laboratory Tests
In addition to specific antidote treatment with fomepizole, patients intoxicated with ethylene glycol or methanol must be managed for metabolic acidosis, acute renal failure (ethylene glycol), adult respiratory distress syndrome, visual disturbances (methanol), and hypocalcemia. Fluid therapy and sodium bicarbonate administration are potential supportive therapies. In addition, potassium and calcium supplementation and oxygen administration are usually necessary. Hemodialysis is necessary in the anuric patient, or in patients with severe metabolic acidosis or azotemia (see DOSAGE AND ADMINISTRATION). Treatment success should be assessed by frequent measurements of blood gases, pH, electrolytes, BUN, creatinine, and urinalysis, in addition to other laboratory tests as indicated by individual patient conditions. At frequent intervals throughout the treatment, patients poisoned with ethylene glycol should be monitored for ethylene glycol concentrations in serum and urine, and the presence of urinary oxalate crystals. Similarly, serum methanol concentrations should be monitored in patients poisoned with methanol.
Electrocardiography should be performed because acidosis and electrolyte imbalances can affect the cardiovascular system. In the comatose patient, electroencephalography may also be required. In addition, hepatic enzymes and white blood cell counts should be monitored during treatment, as transient increases in serum transaminase concentrations and eosinophilia have been noted with repeated fomepizole dosing.Drug Interactions
Oral doses of fomepizole (10 to 20 mg/kg), via alcohol dehydrogenase inhibition, significantly reduced the rate of elimination of ethanol (by approximately 40%) given to healthy volunteers in moderate doses. Similarly, ethanol decreased the rate of elimination of fomepizole (by approximately 50%) by the same mechanism.
Reciprocal interactions may occur with concomitant use of fomepizole and drugs that increase or inhibit the cytochrome P450 system (e.g., phenytoin, carbamazepine, cimetidine, ketoconazole), though this has not been studied.Carcinogenesis & Mutagenesis & Impairment Of Fertility
There have been no long-term studies performed in animals to evaluate carcinogenic potential.
There was a positive Ames test result in the Escherichia coli tester strain WP2uvrA and the Salmonella typhimurium tester strain TA102 in the absence of metabolic activation. There was no evidence of a clastogenic effect in the in vivo mouse micronucleus assay.
In rats, fomepizole (110 mg/kg) administered orally for 40 to 42 days resulted in decreased testicular mass (approximately 8% reduction). This dose is approximately 0.6 times the human maximum daily exposure based on surface area (mg/m2). This reduction was similar for rats treated with either ethanol or fomepizole alone. When fomepizole was given in combination with ethanol, the decrease in testicular mass was significantly greater (approximately 30% reduction) compared to those rats treated exclusively with fomepizole or ethanol.Pregnancy
Pregnancy Category C : Animal reproduction studies have not been conducted with fomepizole. It is also not known whether fomepizole can cause fetal harm when administered to pregnant women or can affect reproduction capacity. Fomepizole should be given to pregnant women only if clearly needed.
-
ADVERSE REACTIONS
The most frequent adverse events reported as drug-related or unknown relationship to study drug in the 78 patients and 63 normal volunteers who received fomepizole injection were headache (14%), nausea (11%), and dizziness, increased drowsiness, and bad taste/metallic taste (6% each). All other adverse events in this population were reported in approximately 3% or fewer of those receiving fomepizole and were as follows:
Body as a Whole : Abdominal pain, fever, multiorgan system failure, pain during fomepizole injection, inflammation at injection site, lumbalgia/backache, hangover.
Cardiovascular : Sinus bradycardia/bradycardia, phlebosclerosis, tachycardia, phlebitis, shock, hypotension.
Gastrointestinal : Vomiting, diarrhea, dyspepsia, heartburn, decreased appetite, transient transaminitis.
Hemic/Lymphatic : Eosinophilia/hypereosinophilia, lymphangitis, disseminated intravascular coagulation, anemia.
Nervous : Lightheadedness, seizure, agitation, feeling drunk, facial flush, vertigo, nystagmus, anxiety, "felt strange", decreased environmental awareness.
Respiratory : Hiccups, pharyngitis.
Skin/Appendages : Application site reaction, rash.
Special Senses : Abnormal smell, speech/visual disturbances, transient blurred vision, roar in ear.
Urogenital : Anuria -
OVERDOSAGE
Nausea, dizziness, and vertigo were noted in healthy volunteers receiving 50 and 100 mg/kg doses of fomepizole (at plasma concentrations of 290 to 520 µmol/L, 23.8 to 42.6 mg/L). These doses are 3 to 6 times the recommended dose. This dose-dependent CNS effect was short-lived in most subjects and lasted up to 30 hours in one subject.
Fomepizole is dialyzable, and hemodialysis may be useful in treating cases of overdosage. -
DOSAGE & ADMINISTRATION
Treatment Guidelines : If ethylene glycol or methanol poisoning is left untreated, the natural progression of the poisoning leads to accumulation of toxic metabolites, including glycolic and oxalic acids (ethylene glycol intoxication) and formic acid (methanol intoxication). These metabolites can induce metabolic acidosis, nausea/vomiting, seizures, stupor, coma, calcium oxaluria, acute tubular necrosis, blindness, and death. The diagnosis of these poisonings may be difficult because ethylene glycol and methanol concentrations diminish in the blood as they are metabolized to their respective metabolites. Hence, both ethylene glycol and methanol concentrations and acid base balance, as determined by serum electrolyte (anion gap) and/or arterial blood gas analysis, should be frequently monitored and used to guide treatment.
Treatment consists of blocking the formation of toxic metabolites using inhibitors of alcohol dehydrogenase, such as fomepizole, and correction of metabolic abnormalities. In patients with high ethylene glycol or methanol concentrations (≥ 50 mg/dL), significant metabolic acidosis, or renal failure, hemodialysis should be considered to remove ethylene glycol or methanol and the respective toxic metabolites of these alcohols.
Treatment with fomepizole :Begin fomepizole treatment immediately upon suspicion of ethylene glycol or methanol ingestion based on patient history and/or anion gap metabolic acidosis, increased osmolar gap, visual disturbances, or oxalate crystals in the urine, OR a documented serum ethylene glycol or methanol concentration greater than 20 mg/dL.
Hemodialysis :Hemodialysis should be considered in addition to fomepizole in the case of renal failure, significant or worsening metabolic acidosis, or a measured ethylene glycol or methanol concentration of greater than or equal to 50 mg/dL. Patients should be dialyzed to correct metabolic abnormalities and to lower the ethylene glycol concentrations below 50 mg/dL.
Discontinuation of fomepizole Treatment :Treatment with fomepizole may be discontinued when ethylene glycol or methanol concentrations are undetectable or have been reduced below 20 mg/dL, and the patient is asymptomatic with normal pH.
Dosing of fomepizole :A loading dose of 15 mg/kg should be administered, followed by doses of 10 mg/kg every 12 hours for 4 doses, then 15 mg/kg every 12 hours thereafter until ethylene glycol or methanol concentrations are undetectable or have been reduced below 20 mg/dL, and the patient is asymptomatic with normal pH. All doses should be administered as a slow intravenous infusion over 30 minutes (see Administration).
Dosage with Renal Dialysis : Fomepizole Injection is dialyzable and the frequency of dosing should be increased to every 4 hours during hemodialysis.
Fomepizole Dosing in Patients Requiring Hemodialysis
DOSE AT THE BEGINNING OF HEMODIALYSIS If <6 hours since last fomepizole dose If ≥ 6 hours since last fomepizole dose Do not administer dose Administer next scheduled dose
DOSING DURING HEMODIALYSIS Dose every 4 hours
DOSING AT THE TIME HEMODIALYSIS IS COMPLETED Time between last dose and the end of
hemodialysis<1 hour Do not administer dose at the end of
hemodialysis1-3 hours Administer 1/2 of next scheduled dose >3 hours Administer next scheduled dose
MAINTENANCE DOSING OFF HEMODIALYSIS Give next scheduled dose 12 hours from last dose administered
Administration : Fomepizole solidifies at temperatures less than 25°C (77°F). If the fomepizole solution has become solid in the vial, the solution should be liquefied by running the vial under warm water or by holding in the hand. Solidification does not affect the efficacy, safety, or stability of fomepizole. Using sterile technique, the appropriate dose of fomepizole should be drawn from the vial with a syringe and injected into at least 100 mL of sterile 0.9% sodium chloride injection or dextrose 5% injection. Mix well. The entire contents of the resulting solution should be infused over 30 minutes. Fomepizole, like all parenteral products, should be inspected visually for particulate matter prior to administration.Stability : Fomepizole diluted in 0.9% sodium chloride injection or dextrose 5% injection remains stable and sterile for at least 24 hours when stored refrigerated or at room temperature. Fomepizole does not contain preservatives. Therefore, maintain sterile conditions, and after dilution do not use beyond 24 hours.
Solutions showing haziness, particulate matter, precipitate, discoloration, or leakage should not be used.
-
HOW SUPPLIED
Fomepizole Injection is available as a sterile, preservative-free solution for intravenous use, in vials containing 1.5mL (1 g/mL) of fomepizole.
Fomepizole injection is supplied in cartons of one single use vial (NDC: 0781-3182-73). or four individual cartons placed into one outer carton (NDC: 0781-3182-84).
Store at controlled room temperature, 20° to 25°C (68° to 77°F).
Manufactured By:
Emcure Pharmaceuticals Ltd.
Pune 411 057, INDIA for:
Sandoz Inc.
Princeton, NJ 08540 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FOMEPIZOLE INJECTION
1.5g/1.5mL (1g/mL)
Rx Only
Single use vial
NDC: 0781-3182-73
For intravenous infusion only
Caution: Must be diluted before use.
Store at controlled room temperature, 20°-25°C (68°-77°F) [see USP].
Manufactured by: Emcure Pharmaceuticals Ltd.
for: Sandoz Inc., Princeton, NJ 08540

-
PACKAGE CARTON PRINCIPAL DISPLAY PANEL
FOMEPIZOLE INJECTION
1.5g/1.5mL (1g/mL)
Rx Only
One single use vial
NDC: 0781-3182-73
For intravenous infusion only
Caution: Must be diluted before use.
Contains: One 1.5mL (1g/mL) vial of Fomepizole Injection.
Each mL contains: 1g Fomepizole
Dosage and Administration: See package insert for dosage and administration information.
Store at controlled room temperature, 20°-25°C (68°-77°F) [see USP].
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Manufactured by: Emcure Pharmaceuticals Ltd.
for: Sandoz Inc., Princeton, NJ 08540

-
PACKAGE OUTER CARTON PRINCIPAL DISPLAY PANEL
FOMEPIZOLE INJECTION
1.5g/1.5mL (1g/mL)
Rx Only
Four single use vials
NDC: 0781-3182-84
For intravenous infusion only
Caution: Must be diluted before use.
Contains: Four1.5mL (1g/mL) vials of Fomepizole Injection.
Each mL contains: 1g Fomepizole
Dosage and Administration: See package insert for dosage and administration information.
Store at controlled room temperature, 20°-25°C (68°-77°F) [see USP].
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Manufactured by: Emcure Pharmaceuticals Ltd.
for: Sandoz Inc., Princeton, NJ 08540
-
INGREDIENTS AND APPEARANCE
FOMEPIZOLE
fomepizole injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0781-3182 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOMEPIZOLE (UNII: 83LCM6L2BY) (FOMEPIZOLE - UNII:83LCM6L2BY) FOMEPIZOLE 1 g in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0781-3182-84 4 in 1 CARTON 1 NDC: 0781-3182-73 1 in 1 CARTON 1 1.5 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078537 03/06/2008 Labeler - Sandoz Inc. (110342024) Establishment Name Address ID/FEI Business Operations Emcure Pharmaceuticals Ltd. 916921919 MANUFACTURE, ANALYSIS
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.