EDLUAR- zolpidem tartrate tablet
Edluar by
Drug Labeling and Warnings
Edluar by is a Prescription medication manufactured, distributed, or labeled by Viatris Specialty LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EDLUAR safely and effectively. See full prescribing information for EDLUAR.
EDLUAR® (zolpidem tartrate) sublingual tablets, for oral use, CIV
Initial U.S. Approval: 1992WARNING: COMPLEX SLEEP BEHAVIORS
See full prescribing information for complete boxed warning.
Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following use of Edluar. Some of these events may result in serious injuries, including death. Discontinue Edluar immediately if a patient experiences a complex sleep behavior. (4, 5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Edluar, a gamma-aminobutyric acid (GABA) A receptor positive modulator, is indicated for the short-term treatment of insomnia characterized by difficulties with sleep initiation. Zolpidem tartrate has been shown to decrease sleep latency for up to 35 days in controlled clinical studies. (1)
DOSAGE AND ADMINISTRATION
- Use the lowest dose effective for the patient (2.1)
- Recommended dose is 5 mg for women and 5 or 10 mg for men, immediately before bedtime. (2.1)
- Geriatric patients and patients with hepatic impairment: Recommended dose is 5 mg for men and women. (2.2)
- Lower doses of CNS depressants may be necessary when taken concomitantly with Edluar. (2.3)
- Co-administration with CNS depressants: Recommended dose is 5 mg for men and women. (2.3)
- The effect of Edluar may be slowed if taken with or immediately after a meal. (2.4)
- Edluar sublingual tablet should be placed under the tongue, where it will disintegrate. (2.4)
- The tablet should not be swallowed and the tablet should not be taken with water. (2.4)
DOSAGE FORMS AND STRENGTHS
Sublingual tablets: 5 mg and 10 mg. Tablets not scored. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- CNS Depressant Effects: Impairs alertness and motor coordination, including risk of morning impairment. Risk increases with dose and use with other CNS depressants and alcohol. Instruct patients on correct use. (5.2)
- Need to Evaluate for Co-morbid Diagnosis: Reevaluate if insomnia persists after 7 to 10 days of use. (5.3)
- Severe Anaphylactic and Anaphylactoid Reactions: angioedema and anaphylaxis have been reported. Do not rechallenge if such reactions occur. (5.4)
- Abnormal Thinking and Behavioral Changes: Changes including decreased inhibition, bizarre behavior, agitation and depersonalization have been reported. Immediately evaluate any new onset behavioral changes. (5.5)
- Depression: Worsening of depression or suicidal thinking may occur. Prescribe the least amount of tablets feasible to avoid intentional overdose. (5.6)
- Respiratory Depression: Consider this risk before prescribing in patients with compromised respiratory function. (5.7)
- Withdrawal Effects: Symptoms may occur with rapid dose reduction or discontinuation. (5.8, 9.3)
ADVERSE REACTIONS
Most commonly observed adverse reactions were:
- Short term (< 10 nights): Drowsiness, dizziness, and diarrhea
- Long term (28-35 nights): Dizziness and drugged feelings (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-866-444-7799 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CNS-depressants, including alcohol: Possible adverse additive CNS-depressant effects (5.1, 7.1)
- Imipramine: decreased alertness observed (7.1)
- Chlorpromazine: impaired alertness and psychomotor performance observed (7.1)
- Rifampin: combination use may decrease effects (7.2)
- Ketoconazole: combination use may increase effect (7.2)
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause respiratory depression and sedation in neonates with exposure late in third trimester. (8.1)
- Lactation: A lactating woman may pump and discard breast milk during treatment and for 23 hours after Edluar administration. (8.2)
- Pediatric use: Safety and effectiveness not established. Hallucinations (incidence rate 7%) and other psychiatric and/or nervous system adverse reactions were observed frequently in a study of pediatric patients with Attention-Deficit/Hyperactivity Disorder. (5.4, 8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: COMPLEX SLEEP BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults
2.2 Special Populations
2.3 Use with CNS Depressants
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Complex Sleep Behaviors
5.2 CNS Depressant Effects and Next-Day Impairment
5.3 Need to Evaluate for Co-morbid Diagnoses
5.4 Severe Anaphylactic and Anaphylactoid Reactions
5.5 Abnormal Thinking and Behavioral Changes
5.6 Use in Patients with Depression
5.7 Respiratory Depression
5.8 Withdrawal Effects
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 CNS-active Drugs
7.2 Drugs That Affect Drug Metabolism Via Cytochrome P450
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Gender Difference in Pharmacokinetics
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Signs and Symptoms
10.2 Recommended Treatment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Transient Insomnia
14.2 Chronic Insomnia
14.3 Studies Pertinent to Safety Concerns for Sedative/Hypnotic Drugs
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: COMPLEX SLEEP BEHAVIORS
Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following use of Edluar. Some of these events may result in serious injuries, including death. Discontinue Edluar immediately if a patient experiences a complex sleep behavior [see Contraindications (4) and Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
Edluar (zolpidem tartrate) sublingual tablets are indicated for the short-term treatment of insomnia characterized by difficulties with sleep initiation [see Clinical Studies (14)].
The clinical trials performed with zolpidem tartrate in support of efficacy were 4-5 weeks in duration with the final formal assessments of sleep latency performed at the end of treatment.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults
Use the lowest effective dose for the patient. The recommended initial dose is 5 mg for women and either 5 or 10 mg for men, taken only once per night immediately before bedtime with at least 7-8 hours remaining before the planned time of awakening. If the 5 mg dose is not effective, the dose can be increased to 10 mg. In some patients, the higher morning blood levels following use of the 10 mg dose increase the risk of next day impairment of driving and other activities that require full alertness [see Warnings and Precautions (5.1)]. The total dose of Edluar should not exceed 10 mg once daily immediately before bedtime.
The recommended initial doses for women and men are different because zolpidem clearance is lower in women.
2.2 Special Populations
Elderly or debilitated patients may be especially sensitive to the effects of zolpidem tartrate. Patients with hepatic insufficiency do not clear the drug as rapidly as normal subjects. The recommended dose of Edluar in both of these patient populations is 5 mg once daily immediately before bedtime [see Warnings and Precautions (5.1); Use in Specific Populations (8.5)].
2.3 Use with CNS Depressants
Dosage adjustment may be necessary when Edluar is combined with other CNS-depressant drugs because of the potentially additive effects [see Warnings and Precautions (5.1)].
-
3 DOSAGE FORMS AND STRENGTHS
Edluar is available in 5 mg and 10 mg strength tablets for sublingual administration. Tablets are not scored.
Edluar 5 mg sublingual tablets are round white, flat-faced, bevel-edged, with debossed V on one side.
Edluar 10 mg sublingual tablets are round white, flat-faced, bevel-edged, with debossed X on one side.
-
4 CONTRAINDICATIONS
Edluar is contraindicated in patients:
- who have experienced complex sleep behaviors after taking Edluar [see Warnings and Precautions (5.1)].
- with known hypersensitivity to zolpidem. Observed reactions include anaphylaxis and angioedema [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Complex Sleep Behaviors
Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following use of zolpidem. Patients can be seriously injured or injure others during complex sleep behaviors. Such injuries may result in a fatal outcome. Other complex sleep behaviors (e.g., preparing and eating food, making phone calls, or having sex) have also been reported. Patients usually do not remember these events. Post-marketing reports have shown that complex sleep behaviors may occur with zolpidem alone at recommended dosages, with or without the concomitant use of alcohol or other central nervous system (CNS) depressants [see Drug Interactions (7.1)]. Discontinue Edluar immediately if a patient experiences a complex sleep behavior.
5.2 CNS Depressant Effects and Next-Day Impairment
Edluar, like other sedative-hypnotic drugs, CNS depressant effects. Co-administration with other CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants, alcohol) increases the risk of CNS depression. Dosage adjustments of Edluar and of other concomitant CNS depressants may be necessary when Edluar is administered with such agents because of the potentially additive effects. The use of Edluar with other sedative-hypnotics (including other zolpidem products) at bedtime or the middle of the night is not recommended [see Dosage and Administration (2.3)].
The risk of next-day psychomotor impairment, including impaired driving, is increased if Edluar is taken with less than a full night of sleep remaining (7 to 8 hours); if a higher than the recommended dose is taken; if co-administered with other CNS depressants; or if co-administered with other drugs that increase the blood level of zolpidem. Patients should be cautioned against driving and other activities requiring complete mental alertness if Edluar is taken in these circumstances.
Because Edluar can cause drowsiness and a decreased level of consciousness, patients, particularly the elderly, are at higher risk of falls.
5.3 Need to Evaluate for Co-morbid Diagnoses
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative/hypnotic drugs, including zolpidem.
5.4 Severe Anaphylactic and Anaphylactoid Reactions
Cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including zolpidem tartrate. Some patients have had additional symptoms such as dyspnea, throat closing or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the throat, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with Edluar should not be rechallenged with the drug.
5.5 Abnormal Thinking and Behavioral Changes
Abnormal thinking and behavior changes have been reported in patients treated with sedative/hypnotics, including zolpidem. Some of these changes included decreased inhibition (e.g. aggressiveness and extroversion that seemed out of character), bizarre behavior, agitation and depersonalization. Visual and auditory hallucinations have been reported.
In controlled trials of zolpidem tartrate 10 mg taken at bedtime, <1% of adults with insomnia who received zolpidem reported hallucinations. In a clinical trial, 7% of pediatric patients treated with zolpidem tartrate 0.25 mg/kg taken at bedtime reported hallucinations, versus 0% treated with placebo [see Use in Specific Populations (8.4)].
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
5.6 Use in Patients with Depression
In primarily depressed patients treated with sedative-hypnotics, worsening of depression, and suicidal thoughts and actions (including completed suicides), have been reported. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in this group of patients; therefore, the least amount of drug that is feasible should be prescribed for the patient at any one time.
5.7 Respiratory Depression
Although studies with 10 mg zolpidem tartrate did not reveal respiratory depressant effects at hypnotic doses in healthy subjects or in patients with mild-to-moderate chronic obstructive pulmonary disease (COPD), a reduction in the Total Arousal Index, together with a reduction in lowest oxygen saturation and increase in the time of oxygen desaturation below 80% and 90%, was observed in patients with mild-to-moderate sleep apnea when treated with zolpidem compared to placebo. Since sedative-hypnotics have the capacity to depress respiratory drive, precautions should be taken if Edluar is prescribed to patients with compromised respiratory function. Post-marketing reports of respiratory insufficiency in patients receiving 10 mg of zolpidem tartrate, most of whom had pre-existing respiratory impairment, have been reported. The risks of respiratory depression should be considered prior to prescribing Edluar in patients with respiratory impairment including sleep apnea and myasthenia gravis.
5.8 Withdrawal Effects
There have been reports of withdrawal signs and symptoms following the rapid dose decrease or abrupt discontinuation of zolpidem. Monitor patients for tolerance, abuse, and dependence [see Drug Abuse and Dependence (9.2) and (9.3)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Complex Sleep Behaviors [see Warnings and Precautions (5.1)]
- CNS-Depressant Effects and Next-Day Impairment [see Warnings and Precautions (5.2)]
- Serious Anaphylactic and Anaphylactoid Reactions [see Warning and Precautions (5.4)]
- Abnormal Thinking and Behavioral Changes [see Warning and Precautions (5.5)]
- Withdrawal Effects [see Warning and Precautions (5.8)]
6.1 Clinical Trials Experience
Associated with discontinuation of treatment:
Approximately 4% of 1,701 patients who received zolpidem tartrate at all doses (1.25 to 90 mg) in U.S. premarketing clinical trials discontinued treatment because of an adverse reaction. Reactions most commonly associated with discontinuation from U.S. trials were daytime drowsiness (0.5%), dizziness (0.4%), headache (0.5%), nausea (0.6%), and vomiting (0.5%).
Approximately 4% of 1,959 patients who received zolpidem tartrate at all doses (1 to 50 mg) in similar foreign trials discontinued treatment because of an adverse reaction. Reactions most commonly associated with discontinuation from these trials were daytime drowsiness (1.1%), dizziness/vertigo (0.8%), amnesia (0.5%), nausea (0.5%), headache (0.4%), and falls (0.4%).
Data from a clinical study in which selective serotonin reuptake inhibitor (SSRI)-treated patients were given zolpidem tartrate revealed that four of the seven discontinuations during double-blind treatment with zolpidem (n=95) were associated with impaired concentration, continuing or aggravated depression, and manic reaction; one patient treated with placebo (n=97) was discontinued after an attempted suicide.
Most commonly observed adverse reactions in controlled trials:
During short-term treatment (up to 10 nights) with zolpidem tartrate at doses up to 10 mg, the most commonly observed adverse reactions associated with the use of zolpidem and seen at statistically significant differences from placebo-treated patients were drowsiness (reported by 2% of zolpidem patients), dizziness (1%), and diarrhea (1%). During longer-term treatment (28 to 35 nights) with zolpidem tartrate at doses up to 10 mg, the most commonly observed adverse reactions associated with the use of zolpidem and seen at statistically significant differences from placebo-treated patients were dizziness (5%) and drugged feelings (3%).
Adverse reactions observed at an incidence of ≥1% in controlled trials:
The following tables enumerate treatment-emergent adverse event frequencies that were observed at an incidence equal to 1% or greater among patients with insomnia who received zolpidem tartrate and at a greater incidence than placebo in U.S. placebo-controlled trials. Events reported by investigators were classified utilizing a modified World Health Organization (WHO) dictionary of preferred terms for the purpose of establishing event frequencies. The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice, in which patient characteristics and other factors differ from those that prevailed in these clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigators involving related drug products and uses, since each group of drug trials is conducted under a different set of conditions. However, the cited figures provide the physician with a basis for estimating the relative contribution of drug and nondrug factors to the incidence of side effects in the population studied.
The following table was derived from a pool of 11 placebo-controlled short-term U.S. efficacy trials involving zolpidem in doses ranging from 1.25 to 20 mg. The table is limited to data from doses up to and including 10 mg, the highest dose recommended for use.
TABLE 1: Incidence of Treatment-Emergent Adverse Experiences in Placebo-Controlled Clinical Trials with zolpidem tartrate lasting up to 10 nights (Percentage of patients reporting) *Reactions reported by at least 1% of patients treated with oral zolpidem and at a greater frequency than placebo. Body System/
Adverse Event*Zolipidem tartrate
(≤ 10 mg)
(N=685)Placebo
(N=473)Central and Peripheral Nervous System
Headache
7
6
Drowsiness
2
-
Dizziness
1
-
Gastrointestinal System
Diarrhea
1
-
The following table was derived from a pool of three placebo-controlled long-term efficacy trials involving oral zolpidem. These trials involved patients with chronic insomnia who were treated for 28 to 35 nights with zolpidem at doses of 5, 10, or 15 mg. The table is limited to data from doses up to and including 10 mg, the highest dose recommended for use. The table includes only adverse events occurring at an incidence of at least 1% for zolpidem patients.
TABLE 2: Incidence of Treatment-Emergent Adverse Experiences in Placebo-Controlled Clinical Trials with zolpidem tartrate lasting up to 35 nights (Percentage of patients reporting) *Reactions reported by at least 1% of patients treated with oral zolpidem and at a greater frequency than placebo. Body System/
Adverse Event*Zolpidem tartrate
(≤ 10 mg)
(N=152)Placebo
(N=161)Autonomic Nervous System
3
1
Dry mouth
Body as a WholeAllergy
4
1
Back Pain
3
2
Influenza-like symptoms
2
-
Chest pain
1
-
Cardiovascular SystemPalpitation
2
-
Central and Peripheral Nervous SystemDrowsiness
8
5
Dizziness
5
1
Lethargy
3
1
Drugged feeling
3
-
Lightheadedness
2
1
Depression
2
1
Abnormal dreams
1
-
Amnesia
1
-
Sleep disorder
1
-
Gastrointestinal SystemDiarrhea
3
2
Abdominal pain
2
2
Constipation
2
1
Respiratory SystemSinusitis
4
2
Pharyngitis
3
1
Skin and AppendagesRash
2
1
Dose relationship for adverse reactions associated with oral zolpidem:
There is evidence from dose comparison trials suggesting a dose relationship for many of the adverse reactions associated with oral zolpidem use, particularly for certain CNS and gastrointestinal adverse events.
Oral tissue-related adverse reactions to Edluar:
The effect of chronic daily administration of Edluar on oral tissue was evaluated in a 60-day open-label study in 60 insomniac patients. One patient developed transient sublingual erythema, and another transient paresthesia of the tongue.
Adverse event incidence across the entire preapproval oral zolpidem database:
Zolpidem was administered to 3,660 subjects in clinical trials throughout the U.S., Canada, and Europe. Treatment-emergent adverse events associated with clinical trial participation were recorded by clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals experiencing treatment-emergent adverse events, similar types of untoward events were grouped into a smaller number of standardized event categories and classified utilizing a modified World Health Organization (WHO) dictionary of preferred terms.
The frequencies presented, therefore, represent the proportions of the 3,660 individuals exposed to zolpidem, at all doses, who experienced an event of the type cited on at least one occasion while receiving zolpidem. All reported treatment-emergent adverse events are included, except those already listed in the table above of adverse events in placebo-controlled studies, those coding terms that are so general as to be uninformative, and those events where a drug cause was remote. It is important to emphasize that, although the events reported did occur during treatment with zolpidem, they were not necessarily caused by it.
Adverse events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in greater than 1/100 subjects; infrequent adverse events are those occurring in 1/100 to 1/1,000 patients; rare events are those occurring in less than 1/1,000 patients.
Autonomic nervous system: Infrequent: increased sweating, pallor, postural hypotension, syncope.
Rare: abnormal accommodation, altered saliva, flushing, glaucoma, hypotension, impotence, increased saliva, tenesmus.Body as a whole: Frequent: asthenia. Infrequent: edema, falling, fever, malaise, trauma.
Rare: allergic reaction, allergy aggravated, anaphylactic shock, face edema, hot flashes, increased ESR, pain, restless legs, rigors, tolerance increased, weight decrease.Cardiovascular system: Infrequent: cerebrovascular disorder, hypertension, tachycardia.
Rare: angina pectoris, arrhythmia, arteritis, circulatory failure, extrasystoles, hypertension aggravated, myocardial infarction, phlebitis, pulmonary embolism, pulmonary edema, varicose veins, ventricular tachycardia.Central and peripheral nervous system: Frequent: ataxia, confusion, euphoria, headache, insomnia, vertigo.
Infrequent: agitation, anxiety, decreased cognition, detached, difficulty concentrating, dysarthria, emotional lability, hallucination, hypoesthesia, illusion, leg cramps, migraine, nervousness, paresthesia, sleeping (after daytime dosing), speech disorder, stupor, tremor.
Rare: abnormal gait, abnormal thinking, aggressive reaction, apathy, appetite increased, decreased libido, delusion, dementia, depersonalization, dysphasia, feeling strange, hypokinesia, hypotonia, hysteria, intoxicated feeling, manic reaction, neuralgia, neuritis, neuropathy, neurosis, panic attacks, paresis, personality disorder, somnambulism, suicide attempts, tetany, yawning.Gastrointestinal system: Frequent: dyspepsia, hiccup, nausea.
Infrequent: anorexia, constipation, dysphagia, flatulence, gastroenteritis, vomiting.
Rare: enteritis, eructation, esophagospasm, gastritis, hemorrhoids, intestinal obstruction, rectal hemorrhage, tooth caries.Hematologic and lymphatic system: Rare: anemia, hyperhemoglobinemia, leukopenia, lymphadenopathy, macrocytic anemia, purpura, thrombosis.
Immunologic system: Infrequent: infection.
Rare: abscess herpes simplex herpes zoster, otitis externa, otitis media.Liver and biliary system: Infrequent: abnormal hepatic function, increased SGPT.
Rare: bilirubinemia, increased SGOT.Metabolic and nutritional: Infrequent: hyperglycemia, thirst.
Rare: gout, hypercholesterolemia, hyperlipidemia, increased alkaline phosphatase, increased BUN, periorbital edema.Musculoskeletal system: Frequent: arthralgia, myalgia. Infrequent: arthritis.
Rare: arthrosis, muscle weakness, sciatica, tendinitis.Reproductive system: Infrequent: menstrual disorder, vaginitis.
Rare: breast fibroadenosis, breast neoplasm, breast pain.Respiratory system: Frequent: upper respiratory infection. Infrequent: bronchitis, coughing, dyspnea, rhinitis.
Rare: bronchospasm, epistaxis, hypoxia, laryngitis, pneumonia.Skin and appendages: Infrequent: pruritus.
Rare: acne, bullous eruption, dermatitis, furunculosis, injection-site inflammation, photosensitivity reaction, urticaria.Special senses: Frequent: diplopia, vision abnormal. Infrequent: eye irritation, eye pain, scleritis, taste perversion, tinnitus.
Rare: conjunctivitis, corneal ulceration, lacrimation abnormal, parosmia, photopsia.Urogenital system: Frequent: urinary tract infection. Infrequent: cystitis, urinary incontinence.
Rare: acute renal failure, dysuria, micturition frequency, nocturia, polyuria, pyelonephritis, renal pain, urinary retention. -
7 DRUG INTERACTIONS
7.1 CNS-active Drugs
CNS Depressants
Co-administration of zolpidem with other CNS depressants increases the risk of CNS depression [see Warnings and Precautions (5.1, 5.2)]. Zolpidem tartrate was evaluated in healthy volunteers in single-dose interaction studies for several CNS drugs.
Imipramine, Chlorpromazine
Imipramine in combination with zolpidem produced no pharmacokinetic interaction other than a 20% decrease in peak levels of imipramine, but there was an additive effect of decreased alertness. Similarly, chlorpromazine in combination with zolpidem produced no pharmacokinetic interaction, but there was an additive effect of decreased alertness and psychomotor performance [see Clinical Pharmacology (12.3)].
Haloperidol
A study involving haloperidol and zolpidem revealed no effect of haloperidol on the pharmacokinetics or pharmacodynamics of zolpidem. The lack of a drug interaction following single-dose administration does not predict the absence of an effect following chronic administration [see Clinical Pharmacology (12.3)].
Alcohol
An additive adverse effect on psychomotor performance between alcohol and oral zolpidem was demonstrated [see Warnings and Precautions (5.1)].
Sertraline
Concomitant administration of zolpidem and sertraline increases exposure to zolpidem and may increase the pharmacodynamics effect of zolpidem [see Clinical Pharmacology (12.3)].
Fluoxetine
After multiple doses of zolpidem tartrate and fluoxetine an increase in the zolpidem half-life (17%) was observed. There was no evidence of an additive effect in psychomotor performance [see Clinical Pharmacology (12.3)].
7.2 Drugs That Affect Drug Metabolism Via Cytochrome P450
Some compounds known to inhibit CYP3A may increase exposure to zolpidem. The effect of other P450 enzymes on the exposure to zolpidem is not known.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Neonates born to mothers using zolpidem late in the third trimester of pregnancy have been reported to experience symptoms of respiratory depression and sedation (see Clinical Considerations and Data). Published data on the use of zolpidem during pregnancy have not identified a drug-associated risk of and major birth defects (see Data). Oral administration of zolpidem to pregnant rats and rabbits did not indicate a risk for adverse effects on fetal development at clinically relevant doses (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Published data from observational studies, birth registries and case reports on the use of zolpidem during pregnancy have not identified a drug-associated risk of major birth defects.
There are limited postmarketing reports of severe to moderate cases of respiratory depression that occurred after birth in neonates whose mothers had taken zolpidem during pregnancy. These cases required artificial ventilation or intra-tracheal intubation. The majority of neonates recovered within hours to a few weeks after birth once treated.
Zolpidem has been shown to cross the placenta.
Animal Data
Oral administration of zolpidem to pregnant rats during the period of organogenesis at 4, 20, and 100 mg base/kg/day, which are approximately 5, 25, and 120 times the MRHD of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area, caused delayed fetal development (incomplete fetal skeletal ossification) at maternally toxic (ataxia) doses 25 and 120 times the MRHD based on mg/m2 body surface area.
Oral administration of zolpidem to pregnant rabbits during the period of organogenesis at 1, 4, and 16 mg base/kg/day, which are approximately 2.5, 10, and 40 times the MRHD of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area caused embryo-fetal death and delayed fetal development (incomplete fetal skeletal ossification) at a maternally toxic (decreased body weight gain) dose 40 times the MRHD based on mg/m2 body surface area.
Oral administration of zolpidem to pregnant rats from day 15 of gestation through lactation at 4, 20, and 100 mg base/kg/day, which are approximately 5, 25, and 120 times the MRHD of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area, delayed offspring growth and decreased survival at doses 25 and 120 times, respectively, the MRHD based on mg/m2 body surface area.
8.2 Lactation
Risk Summary
Limited data from published literature reports the presence of zolpidem in human milk. There are reports of excess sedation in infants exposed to zolpidem through breastmilk (see Clinical Considerations). There is no information on the effects of zolpidem on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Edluar and any potential adverse effects on the breastfed infant from Edluar or from the underlying maternal condition.
Clinical Considerations
Infants exposed to Edluar through breastmilk should be monitored for excess sedation, hypotonia, and respiratory depression. A lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk during treatment and for 23 hours (approximately 5 elimination half-lives) after Edluar administration in order to minimize drug exposure to a breast fed infant.
8.4 Pediatric Use
Edluar is not recommended for use in children. Safety and effectiveness in pediatric patients have not been established in pediatric patients below the age of 18.
In an 8-week controlled study in 201 pediatric patients (aged 6-17 years) with insomnia associated with attention-deficit/hyperactivity disorder (ADHD), an oral solution of zolpidem tartrate dosed at 0.25mg/kg at bedtime did not decrease sleep latency compared to placebo. Ten patients on zolpidem (7.4%) discontinued treatment due to an adverse reaction.
Psychiatric and nervous system disorders comprised the most frequent (>5%) treatment emergent adverse reactions observed with zolpidem versus placebo and included dizziness (23.5% vs. 1.5%), headache (12.5% vs. 9.2%), and hallucinations reported in 7% of the pediatric patients who received zolpidem; none of the pediatric patients who received placebo reported hallucinations [see Warnings and Precautions (5.4)].
8.5 Geriatric Use
A total of 154 patients in U.S. controlled clinical trials and 897 patients in non-U.S. clinical trials who received oral zolpidem were ≥60 years of age. For a pool of U.S. patients receiving zolpidem tartrate at doses of ≤10 mg or placebo, there were three adverse events occurring at an incidence of at least 3% for zolpidem and for which the zolpidem incidence was at least twice the placebo incidence (i.e., they could be considered drug-related).
Adverse Event
Zolpidem
Placebo
Dizziness
3%
0%
Drowsiness
5%
2%
Diarrhea
3%
1%
A total of 30/1,959 (1.5%) non-U.S. patients receiving zolpidem tartrate reported falls, including 28/30 (93%) who were ≥70 years of age. Of these 28 patients, 23 (82%) were receiving zolpidem doses >10 mg. A total of 24/1,959 (1.2%) non-U.S. patients receiving zolpidem reported confusion, including 18/24 (75%) who were ≥70 years of age. Of these 18 patients, 14 (78%) were receiving zolpidem doses >10 mg.
The dose of Edluar in elderly patients is 5 mg to minimize adverse effects related to impaired motor and/or cognitive performance and unusual sensitivity to sedative/hypnotic drugs [see Dosage and Administration (2), Warnings and Precautions (5)Clinical Pharmacology (12) and Clinical Studies (14)].
8.6 Gender Difference in Pharmacokinetics
Women clear zolpidem tartrate from the body at a lower rate than men, Cmax and AUC parameters of zolpidem were approximately 45% higher at the same dose in female subjects compared with male subjects. Given the higher blood levels of zolpidem tartrate in women compared to men at a given dose, the recommended dose of Edluar for adult women is 5 mg, and the recommended dose for adult men is 5 or 10 mg.
In geriatric patients, clearance of zolpidem is similar in men and women. The recommended dose of Edluar in geriatric patients is 5 mg regardless of gender.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Edluar contains the same active substance, zolpidem tartrate, as zolpidem tartrate oral tablets and is classified as a Schedule IV controlled substance by federal regulation.
9.2 Abuse
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of the drug for non-medical purposes, often in combination with other psychoactive substances. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug effects over time. Tolerance may occur to both desired and undesired effects of drugs and may develop at different rates for different effects.
Addiction is a primary, chronic, neurobiological disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, using a multidisciplinary approach, but relapse is common.
Studies of abuse potential in former drug abusers found that the effects of single doses of zolpidem tartrate 40 mg were similar, but not identical, to diazepam 20 mg, while zolpidem tartrate 10 mg was difficult to distinguish from placebo.
Because persons with a history of addiction to or abuse of, drugs or alcohol are at increased risk for misuse, abuse, and addiction of Edluar, they should be monitored carefully when receiving Edluar or any other hypnotic.
9.3 Dependence
Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Sedative/hypnotics have produced withdrawal signs and symptoms following abrupt discontinuation. These reported symptoms range from mild dysphoria and insomnia to a withdrawal syndrome that may include abdominal and muscle cramps, vomiting, sweating, tremors, and convulsions. The following adverse events which are considered to meet the DSM-III-R criteria for uncomplicated sedative/hypnotic withdrawal were reported during U.S. clinical trials following placebo substitution occurring within 48 hours following last zolpidem tartrate treatment: fatigue, nausea, flushing, lightheadedness, uncontrolled crying, emesis, stomach cramps, panic attack, nervousness, and abdominal discomfort. These reported adverse events occurred at an incidence of 1% or less. However, available data cannot provide a reliable estimate of the incidence, if any, of dependence during treatment at recommended doses. Post-marketing reports of abuse, dependence and withdrawal have been received.
-
10 OVERDOSAGE
10.1 Signs and Symptoms
In postmarketing experience of overdose with zolpidem tartrate alone, or in combination with CNS-depressant agents, impairment of consciousness ranging from somnolence to coma, cardiovascular and/or respiratory compromise, and fatal outcomes have been reported.
10.2 Recommended Treatment
Based on data obtained for zolpidem tartrate, general symptomatic and supportive measures for overdose with Edluar should be used along with immediate gastric lavage where appropriate. Intravenous fluids should be administered as needed. Zolpidem’s sedative/hypnotic effect was shown to be reduced by flumazenil and therefore may be useful; however, flumazenil administration may contribute to the appearance of neurological symptoms (convulsions). As in all cases of drug overdose, respiration, pulse, blood pressure, and other appropriate signs should be monitored and general supportive measures employed. Hypotension and CNS depression should be monitored and treated by appropriate medical intervention. Sedating drugs should be withheld following zolpidem overdosage, even if excitation occurs. The value of dialysis in the treatment of overdosage has not been determined, although hemodialysis studies in patients with renal failure receiving therapeutic doses have demonstrated that zolpidem is not dialyzable.
As with the management of all overdosage, the possibility of multiple drug ingestion should be considered. The physician may wish to consider contacting a poison control center for up-to-date information on the management of hypnotic drug product overdosage.
-
11 DESCRIPTION
Edluar contains zolpidem tartrate, a gamma-aminobutyric acid (GABA) A receptor positive modulator of the imidazopyridine class. Edluar is available in 5 mg and 10 mg strength tablets for sublingual administration.
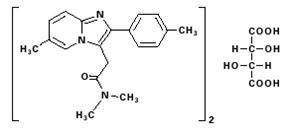
Chemically, zolpidem tartrate is N,N,6-trimethyl-2-p-tolylimidazo[1,2-a] pyridine-3-acetamide L-(+)-tartrate (2:1). It has the following structure:
Zolpidem tartrate is a white to off-white crystalline powder that is sparingly soluble in water, alcohol, and propylene glycol. It has a molecular weight of 764.88.
Each Edluar tablet includes the following inactive ingredients: mannitol, colloidal silicon dioxide, silicified microcrystalline cellulose, croscarmellose sodium, saccharin sodium, and magnesium stearate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zolpidem is a GABA A receptor positive modulator presumed to exert its therapeutic effects in the short-term treatment of insomnia through binding to the benzodiazepine site of α1 subunit containing GABA A receptors, increasing the frequency of chloride channel opening resulting in the inhibition of neuronal excitation.
12.2 Pharmacodynamics
Zolpidem binds to GABAA receptors with a greater affinity for α1 subunit relative to α2 and α3 subunit containing receptors. Zolpidem has no appreciable binding affinity for α5 subunit containing GABAA receptors. This binding profile may explain the relative absence of myorelaxant effects in animal studies. Zolpidem has no appreciable binding affinity for dopaminergic D2, serotonergic 5HT2, adrenergic, histaminergic or muscarinic receptors.
12.3 Pharmacokinetics
Absorption:
Edluar (zolpidem tartrate) sublingual tablets are bioequivalent to Ambien® tablets (Sanofi-Aventis) with respect to Cmax and AUC. Similar to zolpidem tartrate oral tablets, Edluar sublingual tablets result in a pharmacokinetic profile characterized by rapid absorption.
Following administration of single 10 mg Edluar, in 18 healthy adult subjects (18-65 years of age), the mean peak concentration (Cmax) of zolpidem was 106 ng/mL (range: 52 to 205 ng/ml) occurring at a median time (Tmax) of 82 minutes (range: 30-180 min).
A food-effect study in 18 healthy volunteers compared the pharmacokinetics of Edluar 10 mg when administered while fasting or within 20 minutes after a high fat meal. The mean AUC and Cmax were decreased by 20% and 31%, respectively, while median Tmax was prolonged by 28% (from 82 to 105 min). The half-life remained unchanged. These results suggest that, for faster sleep onset, Edluar should not be administered with or immediately after a meal.
Distribution:
Based on data obtained with oral zolpidem, the total protein binding was found to be 92.5 ± 0.1% and remained constant, independent of concentration between 40 and 790 ng/mL.
Metabolism:
Based on data obtained with oral zolpidem, zolpidem is converted to inactive metabolites that are eliminated primarily by renal excretion.
Elimination:
When Edluar administered as a single 5 or 10 mg dose in healthy adult subjects, the mean zolpidem elimination half-life was 2.85 hours (range: 1.57-6.73 hr) and 2.65 hours (range: 1.75 to 3.77 hr) respectively.
Special Populations
Elderly:
In the elderly, the dose for Edluar should be 5 mg [see Warnings and Precautions (5) and Dosage and Administration (2)]. This recommendation is based on several studies with zolpidem tartrate in which the mean Cmax, T1/2, and AUC were significantly increased when compared to results in young adults. In one study of eight elderly subjects (>70 years), the means for Cmax, T1/2, and AUC significantly increased by 50% (255 vs. 384 ng/mL), 32% (2.2 vs. 2.9 hr), and 64% (955 vs. 1,562 nghr/mL), respectively, as compared to younger adults (20 to 40 years) following a single 20 mg oral dose. Zolpidem did not accumulate in elderly subjects following nightly oral dosing of 10 mg for 1 week.
Hepatic Impairment:
The pharmacokinetics of zolpidem tartrate in eight patients with chronic hepatic insufficiency were compared to results in healthy subjects. Following a single 20-mg oral zolpidem tartrate dose, mean Cmax and AUC were found to be two times (250 vs. 499 ng/mL) and five times (788 vs. 4,203 nghr/mL) higher, respectively, in hepatically-compromised patients. Tmax did not change. The mean half-life in cirrhotic patients of 9.9 hr (range: 4.1 to 25.8 hr) was greater than that observed in normals of 2.2 hr (range: 1.6 to 2.4 hr). Dosing with Edluar should be modified accordingly in patients with hepatic insufficiency [see Dosage and Administration (2.2)].
Renal Impairment:
The pharmacokinetics of zolpidem tartrate were studied in 11 patients with end-stage 4 renal failure (mean ClCr = 6.5 ± 1.5 mL/min) undergoing hemodialysis three times a week, who were dosed with zolpidem tartrate 10 mg orally each day for 14 or 21 days. No statistically significant differences were observed for Cmax, Tmax, half-life, and AUC between the first and last day of drug administration when baseline concentration adjustments were made. Zolpidem was not hemodialyzable. No accumulation of unchanged drug appeared after 14 or 21 days. Zolpidem pharmacokinetics were not significantly different in renally-impaired patients. No dosage adjustment is necessary in patients with compromised renal function.
Drug Interactions
CNS-depressants:
Co-administration of zolpidem with other CNS depressants increases the risk of CNS depression [see Warnings and Precautions (5.1)]. Zolpidem tartrate was evaluated in healthy volunteers in single-dose interaction studies for several CNS drugs. Imipramine in combination with zolpidem produced no pharmacokinetic interaction other than a 20% decrease in peak levels of imipramine, but there was an additive effect of decreased alertness. Similarly, chlorpromazine in combination with zolpidem produced no pharmacokinetic interaction, but there was an additive effect of decreased alertness and psychomotor performance.
A study involving haloperidol and zolpidem revealed no effect of haloperidol on the pharmacokinetics or pharmacodynamics of zolpidem. The lack of a drug interaction following single-dose administration does not predict the absence of an effect following chronic administration.
An additive adverse effect on psychomotor performance between alcohol and oral zolpidem was demonstrated [see Warnings and Precautions (5.1)].
Following five consecutive nightly doses at bedtime of oral zolpidem tartrate 10 mg in the presence of sertraline 50 mg (17 consecutive daily doses, at 7:00 am, in healthy female volunteers), zolpidem Cmax was significantly higher (43%) and Tmax was significantly decreased (-53%). Pharmacokinetics of sertraline and N-desmethylsertraline were unaffected by zolpidem.
A single-dose interaction study with zolpidem tartrate 10 mg and fluoxetine 20 mg at steady-state levels in male volunteers did not demonstrate any clinically significant pharmacokinetic or pharmacodynamics interactions. When multiple doses of zolpidem and fluoxetine were given at steady-state and the concentrations evaluated in healthy females, an increase in the zolpidem half-life (17%) was observed. There was no evidence of an additive effect in psychomotor performance.
Drugs that Affect Drug metabolism via Cytochrome P450
Some compounds known to inhibit CYP3A may increase exposure to zolpidem. The effect of inhibitors of other P450 enzymes on the pharmacokinetics of zolpidem is unknown.
A single-dose interaction study with zolpidem tartrate 10 mg and itraconazole 200 mg at steady-state levels in male volunteers resulted in a 34% increase in AUC0-∞ of zolpidem tartrate. There were no pharmacodynamics effects of zolpidem detected on subjective drowsiness, postural sway, or psychomotor performance.
A single-dose interaction study with zolpidem tartrate 10 mg and rifampin 600 mg at steady-state levels in female subjects showed significant reductions of the AUC (-73%), Cmax (-58%), and T½ (-36 %) of zolpidem together with significant reductions in the pharmacodynamics effects of zolpidem tartrate. Rifampin, a CYP3A4 inducer, significantly reduced the exposure to and the pharmacodynamics effects of zolpidem.
A single-dose interaction study with zolpidem 5 mg and ketoconazole, a potent CYP3A4 inhibitor, given as 200 mg twice daily for 2 days increased Cmax of zolpidem (30%) and the total AUC of zolpidem (70%) compared to zolpidem alone and prolonged the elimination half-life (30%) along with an increase in the pharmacodynamics effects of zolpidem. Consideration should be given to using a lower dose of zolpidem when ketoconazole and zolpidem are given together.
Other Drugs with No Interactions with Zolpidem
A study involving cimetidine/zolpidem tartrate and ranitidine/zolpidem tartrate combinations revealed no effect of either drug on the pharmacokinetics or pharmacodynamics of zolpidem.
Zolpidem tartrate had no effect on digoxin pharmacokinetics and did not affect prothrombin time when given with warfarin in healthy subjects.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis:
Zolpidem was administered to mice and rats for 2 years at oral doses of 4, 18, and 80 mg base/kg/day. In mice, these doses are approximately 2.5, 10, and 50 times the maximum recommended human dose (MRHD) of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area and in rats, these doses are approximately 5, 20, and 100 times the MRHD based on mg/m2 body surface area. No evidence of carcinogenic potential was observed in mice. In rats, renal tumors (lipoma, liposarcoma) were seen at the mid and high doses.
Mutagenesis:
Zolpidem was negative in in vitro (bacterial reverse mutation, mouse lymphoma, and chromosomal aberration) and in vivo (mouse micronucleus) genetic toxicology assays.
Impairment of Fertility:
Zolpidem was administered to rats at 4, 20, and 100 mg base/kg/day, which are approximately 5, 25, and 120 times the maximum recommended human dose (MRHD) of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area, prior to and during mating, and continuing in females through postpartum day 25. Zolpidem caused irregular estrus cycles and prolonged precoital intervals at the highest dose tested, which is approximately 120 times the MRHD based on mg/m2 body surface area. The NOAEL for these effects is 25 times the MRHD based on a mg/m2 body surface area. There was no impairment of fertility at any dose tested.
-
14 CLINICAL STUDIES
14.1 Transient Insomnia
Normal adults experiencing transient insomnia (n = 462) during the first night in a sleep laboratory were evaluated in a double-blind, parallel group, single night trial comparing two doses of zolpidem tartrate oral tablets (7.5 and 10 mg) and placebo. Both zolpidem doses were superior to placebo on objective (polysomnographic) measures of sleep latency, sleep duration, and number of awakenings.
Normal elderly adults (mean age 68) experiencing transient insomnia (n = 35) during the first two nights in a sleep laboratory were evaluated in a double-blind, crossover, 2-night trial comparing four doses of zolpidem (5, 10, 15, and 20 mg) and placebo. All zolpidem doses were superior to placebo on the two primary PSG parameters (sleep latency and efficiency) and all four subjective outcome measures (sleep duration, sleep latency, number of awakenings, and sleep quality).
14.2 Chronic Insomnia
Zolpidem was evaluated in two controlled studies for the treatment of patients with chronic insomnia (most closely resembling primary insomnia, as defined in the APA Diagnostic and Statistical Manual of Mental Disorders, DSM-IV™). Adult outpatients with chronic insomnia (n = 75) were evaluated in a double-blind, parallel group, 5-week trial comparing two doses of zolpidem tartrate and placebo. On objective (polysomnographic) measures of sleep latency and sleep efficiency, zolpidem 10 mg was superior to placebo on sleep latency for the first 4 weeks and on sleep efficiency for weeks 2 and 4. Zolpidem was comparable to placebo on number of awakenings at both doses studied.
Adult outpatients (n=141) with chronic insomnia were also evaluated, in a double-blind, parallel group, 4-week trial comparing two doses of zolpidem and placebo. Zolpidem 10 mg was superior to placebo on a subjective measure of sleep latency for all 4 weeks, and on subjective measures of total sleep time, number of awakenings, and sleep quality for the first treatment week.
Increased wakefulness during the last third of the night as measured by polysomnography has not been observed in clinical trials with zolpidem tartrate.
14.3 Studies Pertinent to Safety Concerns for Sedative/Hypnotic Drugs
Next-day residual effects:
Next-day residual effects of zolpidem tartrate were evaluated in seven studies involving normal subjects. In three studies in adults (including one study in a phase advance model of transient insomnia) and in one study in elderly subjects, a small but statistically significant decrease in performance was observed in the Digit Symbol Substitution Test (DSST) when compared to placebo. Studies of zolpidem tartrate in non-elderly patients with insomnia did not detect evidence of next-day residual effects using the DSST, the Multiple Sleep Latency Test (MSLT), and patient ratings of alertness.
Rebound effects:
There was no objective (polysomnographic) evidence of rebound insomnia at recommended doses seen in studies evaluating sleep on the nights following discontinuation of zolpidem tartrate. There was subjective evidence of impaired sleep in the elderly on the first post-treatment night at doses of zolpidem tartrate above the recommended elderly dose of 5 mg.
Memory impairment:
Controlled studies in adults utilizing objective measures of memory yielded no consistent evidence of next-day memory impairment following the administration of zolpidem tartrate. However, in one study involving zolpidem doses of 10 and 20 mg, there was a significant decrease in next-morning recall of information presented to subjects during peak drug effect (90 minutes post-dose), i.e., these subjects experienced anterograde amnesia. There was also subjective evidence from adverse event data for anterograde amnesia occurring in association with the administration of zolpidem tartrate, predominantly at doses above 10 mg.
Effects on sleep stages:
In studies that measured the percentage of sleep time spent in each sleep stage, zolpidem tartrate has generally been shown to preserve sleep stages. Sleep time spent in stages 3 and 4 (deep sleep) was found comparable to placebo with only inconsistent, minor changes in REM (paradoxical) sleep at the recommended dose.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Edluar is supplied as sublingual tablets in two dosage strengths: Tablets are not scored.
Edluar 5 mg sublingual tablets are round white tablets, flat-faced, bevel-edged with debossed V on one side and supplied as:
NDC Number Size
0037-6050-30 blister pack of 30The blister packs consist of aluminum/aluminum Child Resistant Control (CRC) blisters.
Edluar 10 mg sublingual tablets are round white tablets, flat-faced, bevel-edged with debossed X on one side and supplied as:
NDC Number Size
0037-6010-30 blister pack of 30The blister packs consist of aluminum/aluminum Child Resistant Control (CRC) blisters.
Store at controlled room temperature 20-25°C (68-77°F). Protect from light and moisture.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
Inform patients and their families about the benefits and risks of treatment with Edluar. Inform patients of the availability of a Medication Guide and instruct them to read the Medication Guide prior to initiating treatment with Edluar and with each prescription refill. Review the Edluar Medication Guide with every patient prior to initiation of treatment. Instruct patients or caregivers that Edluar should be taken only as prescribed.
Complex Sleep Behaviors
Instruct patients and their families that Edluar may cause complex sleep behaviors, including sleep-walking, sleep-driving, preparing and eating food, making phone calls, or having sex while not being fully awake. Serious injuries and death have occurred during complex sleep behavior episodes. Tell patients to discontinue Edluar and notify their healthcare provider immediately if they develop any of these symptoms [see Boxed Warning, Warnings and Precautions (5.1)].
CNS-depressant Effects and Next-Day Impairment
Tell patients that Edluar has the potential to cause next-day impairment, and that this risk is increased if dosing instructions are not carefully followed. Tell patients to wait for at least 8 hours after dosing before driving or engaging in other activities requiring full mental alertness. Inform patients that impairment can be present despite feeling fully awake. Advise patients that increased drowsiness and decreased consciousness may increase the risk of falls in some patients [see Warnings and Precautions (5.2)].
Severe Anaphylactic and Anaphylactoid Reactions
Inform patients that severe anaphylactic and anaphylactoid reactions have occurred with zolpidem. Describe the signs/symptoms of these reactions and advise patients to seek medical attention immediately if any of them occur [see Warnings and Precautions (5.4)].
Suicide
Tell patients to immediately report any suicidal thoughts.
Alcohol and Other Drugs
Ask patients about alcohol consumption, medicines they are taking, and drugs they may be taking without a prescription. Advise patients not to use Edluar if they drank alcohol that evening or before bed.
Tolerance, Abuse, and Dependence
Tell patients not to increase the dose of Edluar on their own, and to inform you if they believe the drug “does not work”.
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with Edluar. Advise patients that use of Edluar late in the third trimester may cause respiratory depression and sedation in neonates. Advise mothers who used Edluar during the late third trimester of pregnancy to monitor neonates for signs of sleepiness (more than usual), breathing difficulties, or limpness [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding mothers using Edluar to monitor infants for increased sleepiness, breathing difficulties, or limpness. Instruct nursing mothers to seek immediate medical care if they notice these signs. A lactating woman may consider pumping and discarding breastmilk during treatment and for 23 hours after Edluar administration to minimize drug exposure to a breastfed infant [see Use in Specific Populations (8.2)].
Administration Instructions
Patients should be counseled to take Edluar right before they get into bed and only when they are able to stay in bed a full night (7-8 hours) before being active again. Edluar tablets should not be taken with or immediately after a meal. Advise patients NOT to take Edluar when drinking alcohol that evening or before bed. Edluar sublingual tablet should be placed under the tongue, where it will disintegrate. The tablet should not be swallowed and the tablet should not be taken with water.
-
MEDICATION GUIDE
Medication Guide
Edluar® [ED’ – loo-ahr]
(zolpidem tartrate)
sublingual tablets C-IVRead this Medication Guide that comes with Edluar before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about Edluar?
- Do not take more Edluar than prescribed.
- Do not take Edluar unless you are able to stay in bed a full night (7 to 8 hours) before you must be active again.
- Take Edluar right before you get in bed, not sooner.
Edluar may cause serious side effects, including:
-
Complex sleep behaviors that have caused serious injury and death. After taking Edluar, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing (complex sleep behaviors). The next morning, you may not remember that you did anything during the night. These activities may occur with Edluar whether or not you drink alcohol or take other medicines that make you sleepy.
Reported activities include:- o driving a car (“sleep-driving”)
- o making and eating food
- o talking on the phone
- o having sex
- o sleep-walking
Stop taking Edluar and call your healthcare provider right away if you find out that you have done any of the above activities after taking Edluar.
Do not take Edluar if you:
- have ever experienced a complex sleep behavior (such as driving a car, making or eating food, talking on the phone, or having sex) while not being fully awake after taking Edluar
- drank alcohol that evening or before bed
- took another medicine to help you sleep
What is Edluar?
Edluar is a sedative-hypnotic (sleep) medicine. Edluar is used in adults for the short-term treatment of a sleep problem called insomnia (trouble falling asleep).
It is not known if Edluar is safe and effective in children under the age of 18 years.
Edluar is a class four (C-IV) federally controlled substance because it can be abused or lead to dependence. Keep Edluar in a safe place to prevent misuse and abuse. Selling or giving away Edluar may harm others, and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
Who should not take Edluar?
- Do not take Edluar if you are allergic to zolpidem or any other ingredients in Edluar. See the end of this Medication Guide for a complete list of ingredients in Edluar.
- Do not take Edluar if you have had an allergic reaction to drugs containing zolpidem, such as Ambien, Ambien CR, Zolpimist, or Intermezzo.
Symptoms of a serious allergic reaction to zolpidem can include:
- swelling of your face, lips, and throat that may cause difficulty breathing or swallowing
- nausea and vomiting
What should I tell my healthcare provider before taking Edluar?
Edluar may not be right for you. Before starting Edluar, tell your healthcare provider about all of your health conditions, including if you:
- have a history of depression, mental illness or, suicidal thoughts
- have a history of drug or alcohol abuse or addiction
- have kidney or liver disease
- have lung disease or breathing problems
- are pregnant or planning to become pregnant. Talk to your healthcare provider about the risk to your unborn baby if you take Edluar.
- using Edluar in the last trimester of pregnancy may cause breathing difficulties or excess sleepiness in your newborn. Monitor for signs of sleepiness (more than usual), trouble breathing, or limpness in the newborn if Edluar is taken late in pregnancy.
- are breastfeeding or plan to breastfeed. Edluar passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while you take Edluar.
Tell your healthcare provider about all of the medicines you take, including prescription and nonprescription medicines, vitamins and herbal supplements.
Medicines can interact with each other, sometimes causing serious side effects.
Do not take Edluar with other medicines that can make you sleepy, unless directed by your healthcare provider.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist each time you get a new medicine.
How should I take Edluar?
- See “What is the most important information I should know about Edluar?”
- Take Edluar exactly as prescribed. Only take 1 Edluar tablet a night and only if needed.
- Do not take Edluar if you drank alcohol that evening or before bed.
- You should not take Edluar with or right after a meal. Edluar may help you fall asleep faster if you take it on an empty stomach.
- Do not use the tablet if the seal on the childproof blister pack is broken, or if the blister holding the tablet is broken.
- To open the blister pack, separate the individual blisters at the perforations. Peel off the top layer of paper, and push the tablet through the foil. Alternatively, use scissors to open the blister.
- Place the tablet under the tongue, where it will disintegrate. Do not swallow or take with water.
- Call your healthcare provider if your insomnia worsens or is not better within 7 to 10 days. This may mean that there is another condition causing your sleep problem.
- If you take too much Edluar or overdose, get emergency treatment.
What are the possible side effects of Edluar?
Edluar may cause serious side effects, including:
- getting out of bed while not being fully awake and doing an activity that you do not know you are doing. See “What is the most important information I should know about Edluar?”
- abnormal thoughts and behavior. Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, hallucinations, worsening of depression, suicidal thoughts or actions.
- memory loss
- anxiety
- severe allergic reactions. Symptoms include swelling of the tongue or throat, trouble breathing. Get emergency medical help if you get these symptoms after taking Edluar.
Call your healthcare provider right away if you have any of the above side effects or any other side effects that worry you while using Edluar.
The most common side effects of Edluar are:
- drowsiness
- dizziness
- diarrhea
- grogginess or feeling as if you have been drugged
- fatigue
- headache
You may still feel drowsy the next day after taking Edluar.
After you stop taking a sleep medicine, you may have symptoms for 1 to 2 days such as:
- trouble sleeping
- nausea
- flushing
- lightheadedness
- uncontrolled crying
- vomiting
- stomach cramps
- panic attack
- nervousness
- stomach area pain
These are not all the side effects of Edluar. Ask your doctor or pharmacist for more information.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Edluar?
- Store Edluar at room temperature, between 68°F and 77°F (20° to 25°C).
- Protect from light and moisture.
Keep Edluar and all medicines out of reach of children.
General information about the safe and effective use of Edluar
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
Do not use Edluar for a condition for which it was not prescribed.
Do not share Edluar with other people, even if you think they have the same symptoms that you have. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about Edluar.
If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Edluar that is written for healthcare professionals.
For more information about Edluar, go to www.meda.us or call Meda Pharmaceuticals Inc. at 1-866-444-7799.
What are the ingredients in Edluar?
Active Ingredient: zolpidem tartrate
Inactive Ingredients: mannitol, colloidal silicon dioxide, silicified microcrystalline cellulose, croscarmellose sodium, saccharin sodium, and magnesium stearate.
This Medication Guide has been approved by U.S. Food and Drug Administration.
Manufactured by:
Recipharm Stockholm AB, Sweden for
Meda Pharmaceuticals Inc.Distributed by:
Meda Pharmaceuticals Inc.
Somerset, NJ 08873-4120©2019 Mylan Specialty LP
U.S. Patents 6,761,910; 8,512,747; 9,265,720; 9,597,281EDLUAR is a registered trademark of Meda Pharmaceuticals Inc., a Mylan company.
The brands listed are trademarks of their respective owners.
IG-6010-XX4 Rev. XX/2019 797139
-
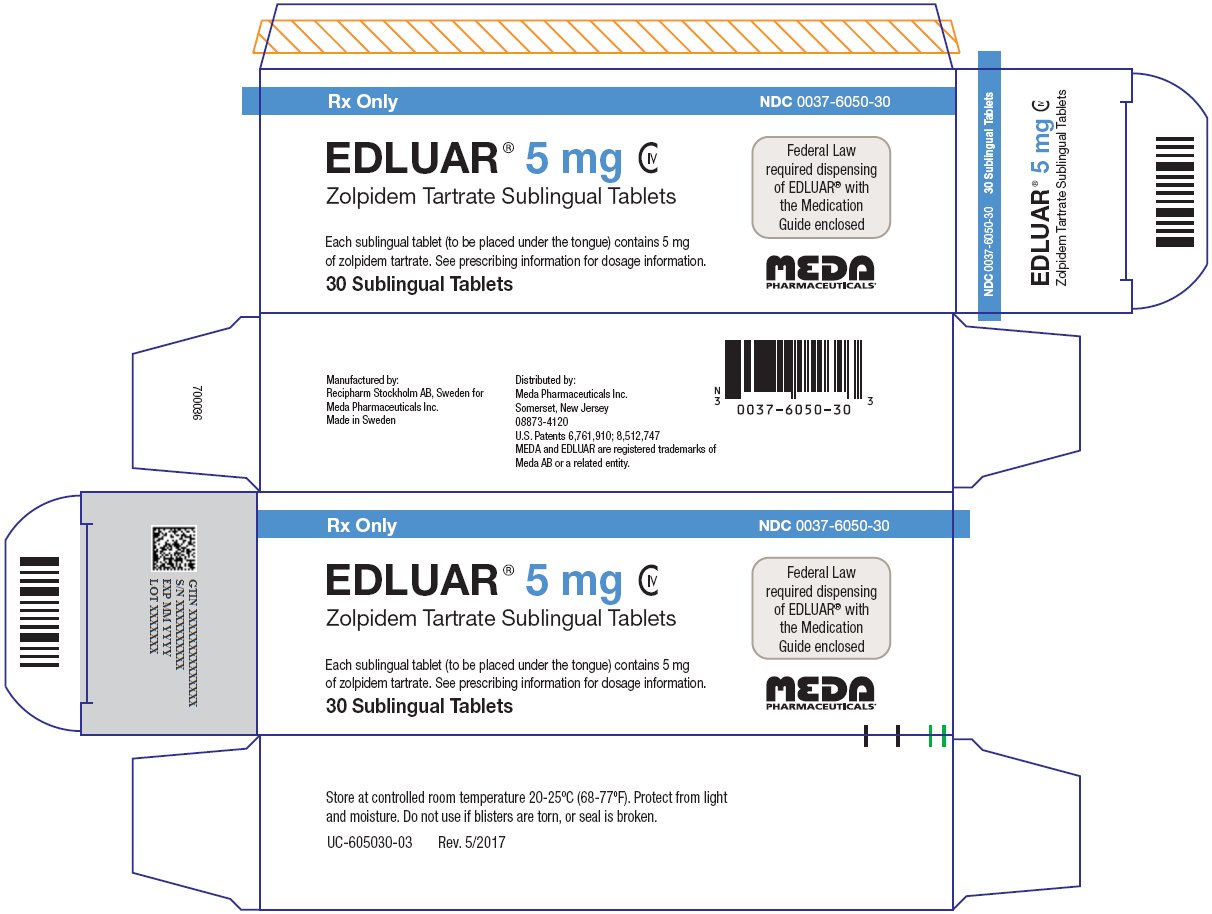
PRINCIPAL DISPLAY PANEL – 5 mg
Rx Only NDC 0037-6050-30
EDLUAR® 5 mg CIV
Zolpidem Tartrate Sublingual TabletsFederal Law
required dispensing
of EDLUAR® with
the Medication
Guide enclosedEach sublingual tablet (to be placed under the tongue) contains 5 mg
of zolpidem tartrate. See prescribing information for dosage information.30 Sublingual Tablets
Store at controlled room temperature 20-25ºC (68-77ºF). Protect from light
and moisture. Do not use if blisters are torn, or seal is broken.UC-605030-03 Rev. 5/2017
Manufactured by:
Recipharm Stockholm AB, Sweden for
Meda Pharmaceuticals Inc.
Made in SwedenDistributed by:
Meda Pharmaceuticals Inc.
Somerset, New Jersey08873-4120
U.S. Patents 6,761,910; 8,512,747
MEDA and EDLUAR are registered trademarks of
Meda AB or a related entity. -
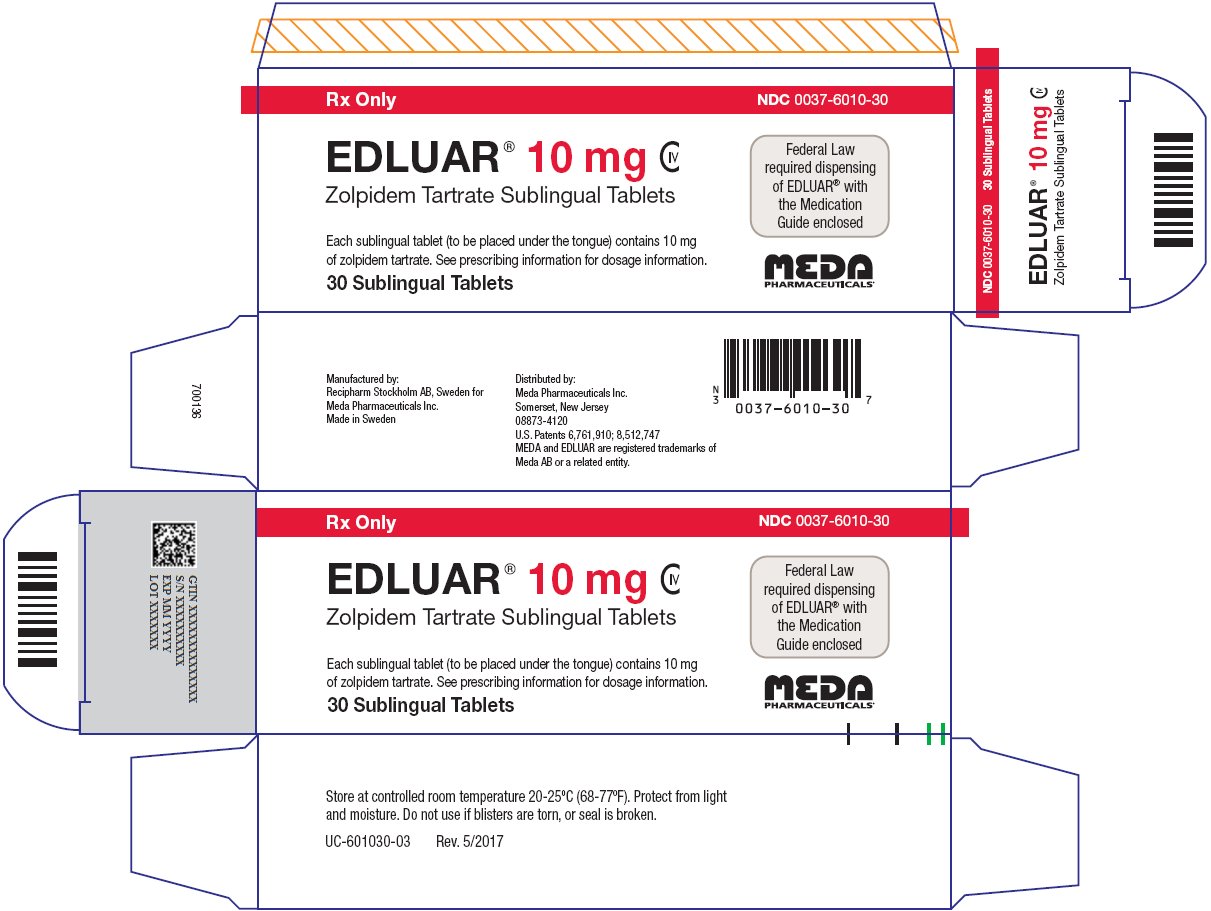
PRINCIPAL DISPLAY PANEL – 10 mg
Rx Only NDC 0037-6010-30
EDLUAR® 10 mg CIV
Zolpidem Tartrate Sublingual TabletsFederal Law
required dispensing
of EDLUAR® with
the Medication
Guide enclosedEach sublingual tablet (to be placed under the tongue) contains 10 mg
of zolpidem tartrate. See prescribing information for dosage information.30 Sublingual Tablets
Store at controlled room temperature 20-25ºC (68-77ºF). Protect from light
and moisture. Do not use if blisters are torn, or seal is broken.UC-601030-03 Rev. 5/2017
Manufactured by:
Recipharm Stockholm AB, Sweden for
Meda Pharmaceuticals Inc.
Made in SwedenDistributed by:
Meda Pharmaceuticals Inc.
Somerset, New Jersey08873-4120
U.S. Patents 6,761,910; 8,512,747
MEDA and EDLUAR are registered trademarks of
Meda AB or a related entity. -
INGREDIENTS AND APPEARANCE
EDLUAR
zolpidem tartrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0037-6050 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZOLPIDEM TARTRATE (UNII: WY6W63843K) (ZOLPIDEM - UNII:7K383OQI23) ZOLPIDEM TARTRATE 5 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SACCHARIN SODIUM (UNII: SB8ZUX40TY) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code V Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0037-6050-30 3 in 1 CARTON 07/24/2009 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021997 07/24/2009 EDLUAR
zolpidem tartrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0037-6010 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZOLPIDEM TARTRATE (UNII: WY6W63843K) (ZOLPIDEM - UNII:7K383OQI23) ZOLPIDEM TARTRATE 10 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SACCHARIN SODIUM (UNII: SB8ZUX40TY) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code X Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0037-6010-30 3 in 1 CARTON 07/24/2009 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 0037-6010-02 20 in 1 BOX, UNIT-DOSE 07/24/2009 2 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021997 07/24/2009 Labeler - Meda Pharmaceuticals (051229602)
Trademark Results [Edluar]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EDLUAR 87946702 5669354 Live/Registered |
Meda Pharmaceuticals Inc. 2018-06-04 |
 EDLUAR 77557224 4045758 Dead/Cancelled |
Meda Pharmaceuticals Inc. 2008-08-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.