Bactine® Max Liquid Bandage
Bactine Max by
Drug Labeling and Warnings
Bactine Max by is a Otc medication manufactured, distributed, or labeled by WellSpring Pharmaceutical Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BACTINE MAX- benzalkonium chloride and lidocaine hydrochloride liquid
WellSpring Pharmaceutical Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Bactine® Max Liquid Bandage
Uses
First aid to help prevent bacterial skin infection and for temporary relief of pain or discomfort in minor cuts, scrapes and burns.
Warnings
For external use only
Do not use
- If you are allergic to any of the ingredients
- in or near the eyes
- over large areas of the body
- longer than 1 week unless directed by a doctor
Directions
- adults and children 2 years of age and older:
- shake well
- clean the affected area and apply a small amount up to 3 times daily and let dry
- a second coating may be applied for extra protection
- children under 2 years of age: ask doctor prior to use
Inactive ingredients
acrylates copolymer, alcohol denat, ammonium acrylates copolymer, disodium deceth-6 sulfosuccinate, disodium EDTA, laureth-30, propanediol, sodium dehydroacetate, water.
Distributed by:
*Germs commonly associated with skin infections.
Distributed by:
WellSpring Pharmaceutical Corporation
Sarasota, FL 34243
© 2022 WellSpring Pharmaceutical Corporation
MONEY BACK GUARANTEE
Bactine Max is FSA/HSA eligible.
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
New
Bactine Max
Liquid Bandage with Lidocaine
Max Pain Relief
Kills 99% of Germs
Prevents Infection
Relieves Kills Prevents
NO STING
SHAKE WELL
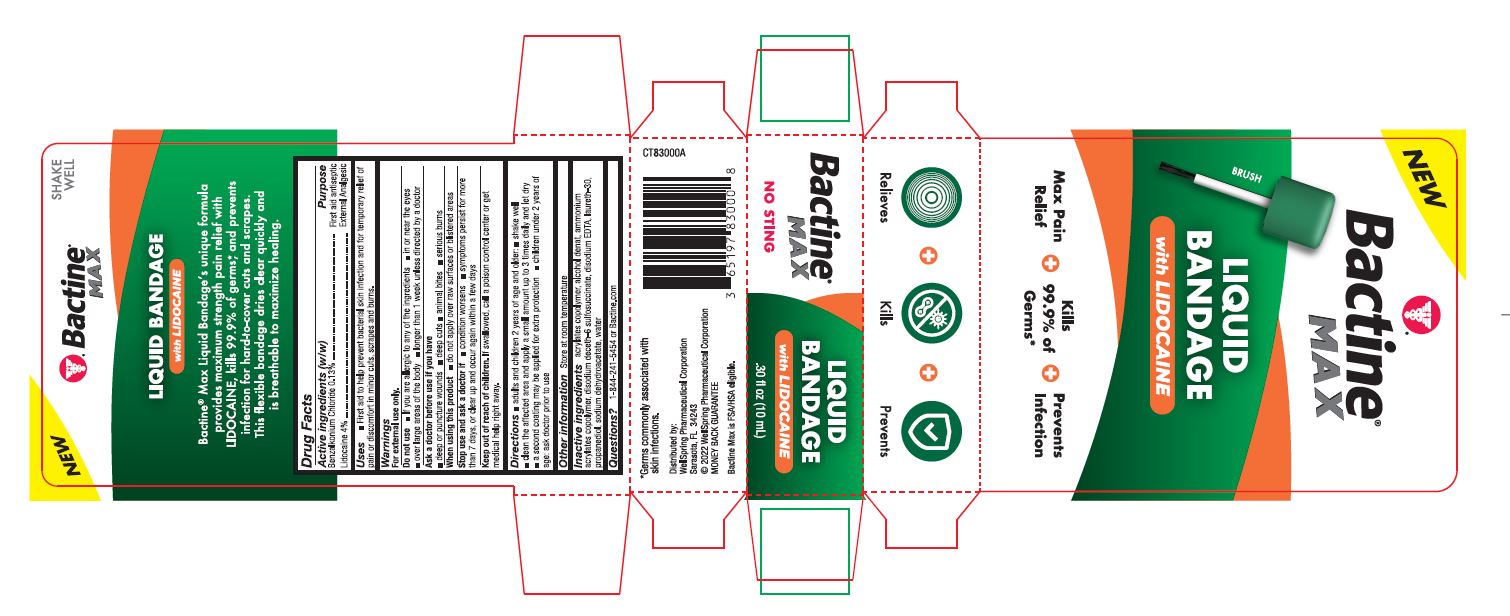
Back Panel
New
Bactine Max
Liquid Bandage with Lidocaine
SHAKE WELL
Bactine Max Liquid Bandageʼs unique formula provides maximum strength pain relief with LIDOCAINE, kills 99.9% of germs*, and prevents infection for hard-to-cover cuts and scrapes. This flexible bandage dries clear quickly and is breathable to maximize healing.
| BACTINE MAX
benzalkonium chloride and lidocaine hydrochloride liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - WellSpring Pharmaceutical Corporation (110999054) |
Trademark Results [Bactine Max]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BACTINE MAX 90017949 not registered Live/Pending |
WellSpring Pharmaceutical Corporation 2020-06-24 |
 BACTINE MAX 88281136 5843473 Live/Registered |
WELLSPRING PHARMACEUTICAL CORPORATION 2019-01-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.