ADSOL RED CELL PRESERVATION SOLUTION SYSTEM IN PLASTIC CONTAINER (PL 146 PLASTIC) (anticoagulant citrate phosphate dextrose- cpd solution and adsol preservation solution kit
ADSOL Red Cell Preservation Solution System in Plastic Container (PL 146 Plastic) by
Drug Labeling and Warnings
ADSOL Red Cell Preservation Solution System in Plastic Container (PL 146 Plastic) by is a Prescription medication manufactured, distributed, or labeled by Fenwal, Inc., Fenwal International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Instructions for Blood Collection Using (CPD) BLOOD-PACK™ Unit with an Integrally Attached Container of ADSOL™ Red Cell Preservation Solution.
Rx Only
BLOOD-PACK™ unit for the collection, processing and/or storage of blood or blood components.
Contains Y-Sampling Site
Use aseptic technique.
CPD formula: Each 63 mL of CPD solution contains: 1.66 g Sodium citrate (dihydrate) USP; 1.61 g Dextrose (monohydrate) USP; 188 mg Citric acid (anhydrous) USP; 140 mg Monobasic sodium phosphate (monohydrate) USP; Water for Injection USP to 63 mL
ADSOL™ formula: Each 100 mL of ADSOL solution contains: 900 mg Sodium chloride USP; 2.20 g Dextrose monohydrate USP; 27.0 mg Adenine USP; 750 mg Mannitol USP; Water for Injection USP to 100 mL.
Caution: Do not use unless the solutions are clear.
- 1. Identify BLOOD-PACK unit using appropriate donor identification system.
- 2. Adjust donor scale to desired collection weight/volume.
- 3. Suspend primary container from donor scale as far as possible below donor arm and clamp donor tubing with hemostat.
- 4. Apply pressure to donor’s arm and disinfect site of venipuncture.
- 5. If blood pressure cuff is used, inflate to approximately 60 mm Hg.
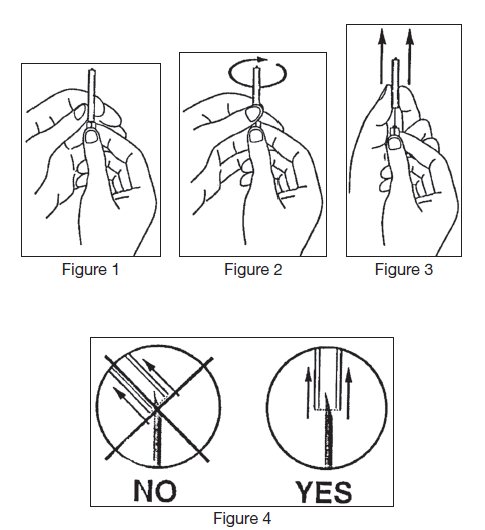
- 6. Remove needle cover per instructions below:
- a) Hold the needle hub upwards. With the other hand, grasp the base of the needle cover (Figure 1), twist approximately 1/4 turn to break tamper evident seal (Figure 2).
- b) Remove needle cover (Figure 3), be careful not to drag cover across the needle point (Figure 4).
- 7. Perform venipuncture, appropriately secure donor needle and/or tubing and release hemostat.
- 8. Mix blood and anticoagulant at several intervals during collection and immediately after collection.
- 9. Collect the appropriate volume based on BLOOD-PACK unit used.
Note: The volume of anticoagulant is sufficient for the blood collection indicated on BLOOD-PACK unit ± 10%.- 10. Release the pressure on the donor’s arm as appropriate.
Precaution: Do not proceed with the remaining steps until the entire whole blood unit is collected.
- 11. To avoid possible contamination of the whole blood unit, before filling whole blood sample tubes, hermetically seal the donor tubing near the Y-Sampling Site on the side leading to the primary container using a metal clip or appropriate alternate method.
Precaution: Complete steps 12 - 20 within approximately 4 minutes after sealing the donor tubing to avoid possible clot formation in the tubing.
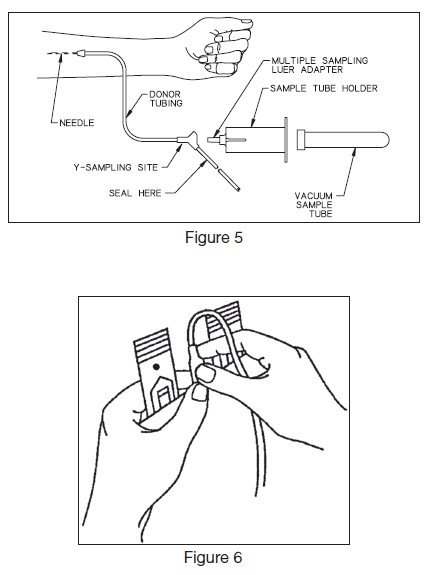
- 12. To collect samples, insert the access device by pushing firmly into the Y-Sampling Site until the membrane seal is penetrated (see Figure 5).
Note: If the access device is assembled such that the outer barrel is screwed onto the Luer, make sure to rotate clockwise upon insertion to avoid barrel detaching from Luer.
- 13. Open the cap on the access device (if applicable).
- 14. Directly align the vacuum sample tube with the internal needle in the access device. Insert vacuum sample tube into device until the stopper is punctured.
- 15. Allow vacuum sample tube to fill with blood then remove from the access device.
- 16. Repeat steps 14 and 15 until the desired number of vacuum sample tubes have been filled.
Notes:
- If the access device needs to be replaced, use a hemostat to clamp the tubing between the needle and the Y-Sampling Site. Then, grasp base of Sampling Site with one hand and pull the access device out with the other hand. Firmly insert the new access device. Remove hemostat and continue sampling.
- If the access device is assembled such that the outer barrel is screwed onto the Luer, make sure to rotate clockwise upon removal to avoid barrel detaching from Luer.
- The access device can only be replaced one time.
Precaution: When replacing the access device, be careful to avoid contact with any blood droplets on the Luer or Sampling Site. Discard used access device appropriately.
- 17. Release remaining pressure on donor’s arm.
- 18. If desired, apply hemostat to donor tubing between needle and Y-Sampling Site.
- 19. Withdraw the needle.
- 20. Preparation of AS-1 Red Blood Cells may vary depending on processing option selected:
- a) After removal of plasma from freshly collected blood.
- b) Within 8 hours of blood collection if whole blood is held at ambient temperature.
- c) Within 3 days of collection if blood is refrigerated immediately following collection.
- 21. Centrifuge primary and secondary containers to prepare CPD Red Blood Cells.
- 22. Place primary container in plasma extractor and express plasma into empty TRANSFER-PACK™ container by releasing pressure plate and breaking cannula. (To break cannula, grasp the base of the cannula with one hand. With the other hand grasp the top of the cannula and bend 90° in one direction then 180° in opposite direction (Figure 6)).
- 23. When desired amount of plasma has been removed, clamp tubing between Y and plasma container.
- 24. Suspend ADSOL™ red cell preservation solution container, break cannula (Figure 6) and drain contents into primary container of CPD Red Blood Cells. Clamp tubing.
- 25. Seal transfer tubing in three places near primary container and cut the middle seal, being careful to avoid fluid splatter. For Double BLOOD-PACK unit codes, discard ADSOL solution container. For other ADSOL codes, the empty solution container may now be used as a TRANSFER-PACK container for further component preparation.
- 26. Mix ADSOL solution and red cells thoroughly.
- 27. Store suspended AS-1 Red Blood Cells between 1 and 6°C.
- 28. Infuse AS-1 Red Blood Cells within 42 days of collection.
For further processing, use standard component processing and storage techniques.
Dispose of containers and materials into appropriate biohazardous waste containers following established procedures.
Single use only. Sterile, non-pyrogenic fluid path. Store at Controlled Room Temperature.
USP Definition of “Controlled Room Temperature”
United States Pharmacopeia, General Notices.
United States Pharmacopeial Convention, Inc.
12601 Twinbrook Parkway, Rockville, MD Manufactured by:
Manufactured by:
Fenwal International, Inc.
Road 357, Km. 0.8
Maricao, PR 00606Made in USA
FENWAL, BLOOD-PACK, ADSOL and TRANSFER-PACK are trademarks of Fenwal, Inc.
07-19-05-702 REV: A 03/2011
© 2011 Fenwal, Inc. All rights reserved.
-
PACKAGE/LABEL DISPLAY PANEL
N4R6346
6 Units
Fenwal™
Triple BLOOD-PACK™ Unit CPD/ADSOL™
PL 146
PL 1240
PlasticRx only
Triple BLOOD-PACK unit consisting of a primary pack containing 63 mL of CPD anticoagulant solution for collection of 450 mL of blood, one TRANSFER-PACK containing 100 mL of ADSOL solution and one TRANSFER-PACK without solution. 16 gauge needle.
See instructions for use. Sterile, non-pyrogenic fluid path. Steam sterilized. Single use only. Do not vent. Do not use if there is any visible sign of deterioration. Dispose of container appropriately. Store at Controlled Room Temperature (refer to direction insert). Unused packs in open pouches may be kept 60 days by folding and securing end of pouch, to prevent loss of moisture.
Direct handling of product surfaces prior to extended storage in the foil pack may result in mold growth.
Units removed from the foil pouch must be used within 4 days (96 hours). Units out of the foil pouch for longer than 4 days must be discarded.
CPD formula: Each 63 mL of CPD solution contains: 1.66 g, Sodium citrate (dihydrate), USP, 1.61 g Dextrose (monohydrate), USP, 188 mg Citric acid (anhydrous), USP, 140 mg Monobasic sodium phosphate (monohydrate), USP. Water for Injection USP to 63 mL
ADSOL formula: Sodium chloride 900 mg, USP, Dextrose monohydrate 2.20 g, USP, Adenine 27.0 mg, USP, Mannitol 750 mg, USP. Water for Injection USP to 100 mL.
 Manufactured by:
Manufactured by:
Fenwal International, Inc.
Road 357, Km. 0.8
Maricao, PR 00606Made in USA
07-28-05-404 REV: A
Imported and distributed in Thailand by:
Fenwal (Thailand) Ltd.
17th Fl. Thanapoom Tower,
1550 New Petchburi Rd., Makasan
Rajthevi, Bangkok 10400
ThailandDate of opening of pouch:
FENWAL, BLOOD-PACK, TRANSFER-PACK and ADSOL are trademarks of Fenwal, Inc.
Lot No.:
Exp. date:

-
INGREDIENTS AND APPEARANCE
ADSOL RED CELL PRESERVATION SOLUTION SYSTEM IN PLASTIC CONTAINER (PL 146 PLASTIC)
anticoagulant citrate phosphate dextrose (cpd) solution and adsol preservation solution kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0942-6501 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0942-6501-03 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BAG 63 mL Part 2 1 BAG 100 mL Part 1 of 2 CPD
citrate phosphate dextrose solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trisodium Citrate Dihydrate (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 1.66 g in 63 mL Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 1.61 g in 63 mL Anhydrous Citric Acid (UNII: XF417D3PSL) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 188 mg in 63 mL Sodium Phosphate, Monobasic, Monohydrate (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) Sodium Phosphate, Monobasic, Monohydrate 140 mg in 63 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 63 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN811104 03/04/2011 Part 2 of 2 ADSOL RED CELL PRESERVATION SOLUTION SYSTEM
adsol red cell preservation solution solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 2.2 g in 100 mL Sodium Chloride (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) Sodium Chloride 900 mg in 100 mL Mannitol (UNII: 3OWL53L36A) (Mannitol - UNII:3OWL53L36A) Mannitol 750 mg in 100 mL Adenine (UNII: JAC85A2161) (Adenine - UNII:JAC85A2161) Adenine 27 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN811104 03/04/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN811104 03/04/2011 Labeler - Fenwal, Inc. (794519020) Establishment Name Address ID/FEI Business Operations Fenwal International, Inc. 091164590 MANUFACTURE(0942-6501)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.