TRI-LUMA- fluocinolone acetonide, hydroquinone, and tretinoin cream
TRI-LUMA by
Drug Labeling and Warnings
TRI-LUMA by is a Prescription medication manufactured, distributed, or labeled by Galderma Laboratories, L.P., Hill Dermaceuticals, Inc., G Production, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRI-LUMA Cream safely and effectively. See full prescribing information for TRI-LUMA Cream.
TRI-LUMA® (fluocinolone acetonide, hydroquinone, tretinoin) cream, 0.01%/4%/0.05% for topical use
Initial U.S. Approval: 2002INDICATIONS AND USAGE
TRI-LUMA Cream is a combination of fluocinolone acetonide (a corticosteroid), hydroquinone (a melanin synthesis inhibitor), and tretinoin (a retinoid) that is indicated for the short-term treatment of moderate to severe melasma of the face, in the presence of measures for sun avoidance, including the use of sunscreens. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Cream, 0.01%/4%/0.05%. Each gram of TRI-LUMA Cream contains 0.1 mg of fluocinolone acetonide, 40 mg of hydroquinone, and 0.5 mg of tretinoin. (3)
CONTRAINDICATIONS
- TRI-LUMA Cream is contraindicated in individuals with a history of hypersensitivity to this product or any of its components. (4)
WARNINGS AND PRECAUTIONS
- TRI-LUMA Cream contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening asthmatic episodes in susceptible people. If anaphylaxis, asthma or other clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue TRI-LUMA. (5.1)
- TRI-LUMA Cream contains hydroquinone, which may produce exogenous ochronosis, a gradual blue-black darkening of the skin, the occurrence of which should prompt discontinuation of therapy. (5.2)
ADVERSE REACTIONS
Most common adverse reactions (incidence > 5%) are erythema, desquamation, burning, dryness, pruritus, and acne. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Galderma Laboratories, L.P. at 1-866-735-4137 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
TRI-LUMA Cream contains the teratogen, tretinoin, which may cause embryofetal death, altered fetal growth, congenital malformations, and potential neurologic deficits. TRI-LUMA Cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Indication
1.2 Limitations of Use
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Exogenous Ochronosis
5.3 Effects on Endocrine System
5.4 Cutaneous Reactions
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Indication
TRI-LUMA Cream is a combination of fluocinolone acetonide (a corticosteroid), hydroquinone (a melanin synthesis inhibitor), and tretinoin (a retinoid) that is indicated for the short-term treatment of moderate to severe melasma of the face, in the presence of measures for sun avoidance, including the use of sunscreens.
1.2 Limitations of Use
TRI-LUMA Cream is NOT indicated for the maintenance treatment of melasma. After achieving control with TRI-LUMA Cream, some patients may be managed with other treatments instead of triple therapy with TRI-LUMA Cream. Melasma usually recurs upon discontinuation of TRI-LUMA Cream.
The safety and efficacy of TRI-LUMA Cream in patients of Fitzpatrick Skin Types V and VI have not been studied. Excessive bleaching resulting in undesirable cosmetic effect in patients with darker skin cannot be excluded.
The safety and efficacy of TRI-LUMA Cream in the treatment of hyperpigmentation conditions other than melasma of the face have not been studied.
Because pregnant and lactating women were excluded from, and women of childbearing potential had to use birth control measures in the clinical trials, the safety and efficacy of TRI-LUMA Cream in pregnant women and nursing mothers have not been established [see Use in Specific Populations (8.1, 8.3)].
-
2 DOSAGE AND ADMINISTRATION
Apply a thin film of TRI-LUMA Cream to the effected area once daily, at least 30 minutes before bedtime.
Gently wash the face and neck with a mild cleanser. Rinse and pat the skin dry. Apply TRI-LUMA Cream to the hyperpigmented areas of melasma including about 1/2 inch of normal appearing skin surrounding each lesion. Rub lightly and uniformly into the skin.
Therapy should be discontinued when control is achieved.
During the day, use a sunscreen of SPF 30, and wear protective clothing. Avoid sunlight exposure. Patients may use moisturizers and/or cosmetics during the day.
TRI-LUMA Cream is for topical use only. It is not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
TRI-LUMA Cream contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening asthmatic episodes in susceptible individuals. If anaphylaxis, asthma or other clinically significant hypersensitivity reactions occur, institute appropriate therapy and discontinue TRI-LUMA. Allergic contact dermatitis may also occur [see Warnings and Precautions 5.4].
5.2 Exogenous Ochronosis
TRI-LUMA Cream contains hydroquinone, which may produce exogenous ochronosis, a gradual blue-black darkening of the skin, the occurrence of which should prompt discontinuation of therapy. The majority of patients developing this condition are Black, but it may also occur in Caucasians and Hispanics.
5.3 Effects on Endocrine System
TRI-LUMA Cream contains the corticosteroid fluocinolone acetonide. Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria can also be produced by systemic absorption of topical corticosteroid while on treatment. If HPA axis suppression is noted, the use of TRI-LUMA Cream should be discontinued. Recovery of HPA axis function generally occurs upon discontinuation of topical corticosteroids.
The ACTH or cosyntropin stimulation test may be helpful in evaluating patients for HPA axis suppression.
5.4 Cutaneous Reactions
Cutaneous hypersensitivity to the active ingredients of TRI-LUMA Cream has been reported in the literature. In a patch test study to determine sensitization potential in 221 healthy volunteers, three volunteers developed sensitivity reactions to TRI-LUMA Cream or its components.
TRI-LUMA Cream contains hydroquinone and tretinoin that may cause mild to moderate irritation. Local irritation, such as skin reddening, peeling, mild burning sensation, dryness, and pruritus may be expected at the site of application. Transient skin reddening or mild burning sensation does not preclude treatment. If a reaction suggests hypersensitivity or chemical irritation, the use of the medication should be discontinued.
Patients should avoid medicated or abrasive soaps and cleansers, soaps and cosmetics with drying effects, products with high concentrations of alcohol and astringents, and other irritants or keratolytic drugs while on TRI-LUMA Cream treatment. Patients are cautioned on concomitant use of medications that are known to be photosensitizing.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In the controlled clinical trials, adverse events were monitored in the 161 subjects who used TRI-LUMA Cream once daily during an 8-week treatment period. There were 102 (63%) subjects who experienced at least one treatment-related adverse event during these trials. The most frequently reported events were erythema, desquamation, burning, dryness, and pruritus at the site of application. The majority of these events were mild to moderate in severity. Adverse events reported by at least 1% of patients and judged by the investigators to be reasonably related to treatment with TRI-LUMA Cream from the controlled clinical trials are summarized (in decreasing order of frequency) as follows:
Table 1. Incidence and Frequency of Treatment-related Adverse Events with TRI-LUMA Cream in at least 1% or more of Subjects (N=161) Adverse Event n (%) Erythema 66 (41%) Desquamation 61 (38%) Burning 29 (18%) Dryness 23 (14%) Pruritus 18 (11%) Acne 8 (5%) Paresthesia 5 (3%) Telangiectasia 5 (3%) Hyperesthesia 3 (2%) Pigmentary changes 3 (2%) Irritation 3 (2%) Papules 2 (1%) Acne-like rash 1 (1%) Rosacea 1 (1%) Dry Mouth 1 (1%) Rash 1 (1%) Vesicles 1 (1%) In an open-label trial, subjects who had cumulative treatment of melasma with TRI-LUMA Cream for 6 months showed a similar pattern of adverse events as in the 8-week studies.
The following local adverse reactions have been reported with topical corticosteroids. They may occur more frequently with the use of occlusive dressings, especially with higher potency corticosteroids. These reactions are listed in an approximate decreasing order of occurrence: burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, and miliaria.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. TRI-LUMA Cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. TRI-LUMA Cream contains the teratogen, tretinoin, which may cause embryo-fetal death, altered fetal growth, congenital malformations, and potential neurologic deficits.
In clinical trials involving TRI-LUMA Cream in the treatment of facial melasma, women of child-bearing potential initiated treatment only after having had a negative pregnancy test and used effective birth control measures during therapy. However, 13 women became pregnant during treatment with TRI-LUMA Cream. Most of the pregnancy outcomes are unknown. Three women gave birth to apparently healthy babies. One pregnancy was terminated prematurely, and another ended in miscarriage.
In general, use of drugs should be reduced to a minimum in pregnancy. If a patient has been inadvertently exposed to TRI-LUMA Cream in pregnancy, she should be counseled on the risk of teratogenesis due to this exposure. The risk of teratogenesis due to topical exposure to TRI-LUMA Cream may be considered low. However, exposure during the period of organogenesis in the first trimester is theoretically more likely to produce adverse outcome than in later pregnancy.
Tretinoin is considered to be highly teratogenic upon systemic administration. Animal reproductive studies are not available with topical hydroquinone. Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
In a dermal application study using TRI-LUMA Cream in pregnant rabbits, there was an increase in the number of in utero deaths and a decrease in fetal weights in litters from dams treated topically with the drug product.
In a dermal application study in pregnant rats treated with TRI-LUMA Cream during organogenesis there was evidence of teratogenicity of the type expected with tretinoin. These morphological alterations included cleft palate, protruding tongue, open eyes, umbilical hernia, and retinal folding or dysplasia.
In a dermal application study on the gestational and postnatal effects of a 10-fold dilution of TRI-LUMA Cream in rats, an increase in the number of stillborn pups, lower pup body weights, and delay in preputial separation were observed. An increase in overall activity was seen in some treated litters at postnatal day 22 and in all treated litters at five weeks, a pattern consistent with effects previously noted in animals exposed in utero with retinoic acids. No adequate study of the late gestational and postnatal effects of the full-strength TRI-LUMA Cream has been performed.
It is difficult to interpret these animal studies on teratogenicity with TRI-LUMA Cream, because the availability of the dermal applications in these studies could not be assured, and comparison with clinical dosing is not possible.
8.3 Nursing Mothers
Corticosteroids, when systemically administered, appear in human milk. It is not known whether topical application of TRI-LUMA Cream could result in sufficient systemic absorption to produce detectable quantities of fluocinolone acetonide, hydroquinone, or tretinoin in human milk. Because many drugs are secreted in human milk, caution should be exercised when TRI-LUMA Cream is administered to a nursing woman. Care should be taken to avoid contact between the infant being nursed and TRI-LUMA Cream.
8.4 Pediatric use
Safety and effectiveness of TRI-LUMA Cream in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of TRI-LUMA Cream did not include sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
TRI-LUMA (fluocinolone acetonide, hydroquinone, and tretinoin) Cream, 0.01%/4%/0.05% contains fluocinolone acetonide, USP, hydroquinone, USP, and tretinoin, USP, in a light yellow, hydrophilic cream base for topical application.
Fluocinolone acetonide is a synthetic fluorinated corticosteroid. It is a white crystalline powder that is odorless and stable in light.
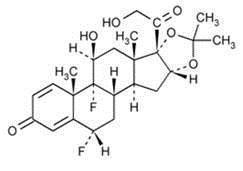
The chemical name for fluocinolone acetonide is: (6α,11β,16α)-6,9-difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-pregna-1,-4-diene-3,20-dione.
The molecular formula is C24H30F2O6 and molecular weight is 452.50.
Fluocinolone acetonide has the following structural formula:
Hydroquinone is a melanin synthesis inhibitor. It is prepared from the reduction of p-benzoquinone with sodium bisulfite. It occurs as fine white needles that darken on exposure to air.
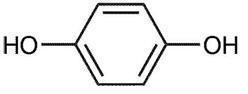
The chemical name for hydroquinone is: 1,4-benzenediol.
The molecular formula is C6H6O2 and molecular weight is 110.11.
Hydroquinone has the following structural formula:
Tretinoin, a retinoid, is all-trans-retinoic acid formed from the oxidation of the aldehyde group of retinene to a carboxyl group. It occurs as yellow to light-orange crystals or crystalline powder with a characteristic odor of ensilage. It is highly reactive to light and moisture.
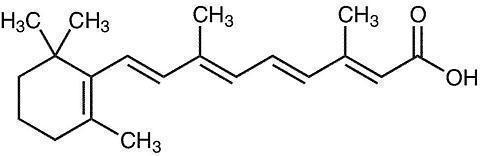
The chemical name for tretinoin is: (all-E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid.
The molecular formula is C20H28O2 and molecular weight is 300.44.
Tretinoin has the following structural formula:
Each gram of TRI-LUMA Cream contains Active: fluocinolone acetonide 0.01% (0.1 mg), hydroquinone 4% (40 mg), and tretinoin 0.05% (0.5 mg). Inactive: butylated hydroxytoluene, cetyl alcohol, citric acid anhydrous, glycerin, glyceryl stearate, magnesium aluminum silicate, methyl gluceth-10, methylparaben, PEG-100 stearate, propylparaben, purified water, sodium metabisulfite, stearic acid, and stearyl alcohol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of the active ingredients in TRI-LUMA Cream in the treatment of melasma is unknown.
12.3 Pharmacokinetics
Percutaneous absorption of unchanged tretinoin, hydroquinone and fluocinolone acetonide into the systemic circulation of two groups of healthy volunteers (Total N=59) was found to be minimal following 8 weeks of daily application of 1g (Group I, n=45) or 6g (Group II, n=14) of TRI-LUMA Cream.
For tretinoin quantifiable plasma concentrations were obtained in 57.78% (26 out of 45) of Group I and 57.14% (8 out of 14) of Group II subjects. The exposure to tretinoin as reflected by the Cmax values ranged from 2.01 to 5.34 ng/mL (Group I) and 2.0 to 4.99 ng/mL (Group II). Thus, daily application of TRI-LUMA Cream resulted in a minimal increase of normal endogenous levels of tretinoin. The circulating tretinoin levels represent only a portion of total tretinoin-associated retinoids, which would include metabolites of tretinoin and that sequestered into peripheral tissues.
For hydroquinone, quantifiable plasma concentrations were obtained in 18% (8 out of 44) Group I subjects. The exposure to hydroquinone, as reflected by the Cmax values, ranged from 25.55 to 86.52 ng/mL. All Group II subjects (6g dose) had post-dose plasma hydroquinone concentrations below the quantitation limit. For fluocinolone acetonide, Groups I and II subjects had all post-dose plasma concentrations below quantitation limit.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
When fluocinolone acetonide, hydroquinone, and tretinoin in fixed combinations equivalent to 10%, 50%, 100%, and 150% of the concentrations in the clinical formulation of TRI-LUMA Cream were applied topically to male and female CD-1 mice for up to 24 months at dosages approximating up to 50, 19,000, and 250 µg/kg/day, respectively (corresponding to dosages of 150, 57,000, and 750 μg/m2/day, respectively), no statistically significant changes in tumor incidence were observed.
When fluocinolone acetonide, hydroquinone, and tretinoin in fixed combinations equivalent to 10%, 25%, 50%, and 100% of the concentrations in the clinical formulation of TRI-LUMA Cream were applied topically to male and female SD rats for up to 24 months at dosages approximating up to 10, 4000, and 50 µg/kg/day, respectively (corresponding to dosages of 60, 24,000, and 300 μg/m2/day, respectively), statistically significant increases in the incidences of islet cell adenomas and combined islet cell adenomas and carcinomas of the pancreas in both males and females were observed. The clinical relevance of these findings is unknown.
Studies of hydroquinone in animals have demonstrated some evidence of carcinogenicity. The carcinogenic potential of hydroquinone in humans is unknown.
Studies in hairless albino mice suggest that concurrent exposure to tretinoin may enhance the tumorigenic potential of carcinogenic doses of UVB and UVA light from a solar simulator. This effect has been confirmed in a later study in pigmented mice, and dark pigmentation did not overcome the enhancement of photocarcinogenesis by 0.05% tretinoin. Although the significance of these studies to humans is not clear, patients should minimize exposure to sunlight or artificial ultraviolet irradiation sources.
Mutagenicity studies were not conducted with this combination of active ingredients. Published studies have demonstrated that hydroquinone is a mutagen and a clastogen. Treatment with hydroquinone has resulted in positive findings for genetic toxicity in the Ames assay in bacterial strains sensitive to oxidizing mutagens, in in vitro studies in mammalian cells, and in the in vivo mouse micronucleus assay. Tretinoin has been shown to be negative for mutagenesis in the Ames assay. Additional information regarding the genetic toxicity potential of tretinoin and of fluocinolone acetonide is not available.
A dermal reproductive fertility study was conducted in SD rats using a 10-fold dilution of the clinical formulation. No effect was seen on the traditional parameters used to assess fertility, although prolongation of estrus was observed in some females, and there was a trend towards an increase in pre-and post-implantation loss that was not statistically significant. No adequate study of fertility and early embryonic toxicity of the full-strength drug product has been performed. In a six-month study in minipigs, small testes and severe hypospermia were found when males were treated topically with the full strength drug product.
-
14 CLINICAL STUDIES
Two adequate and well-controlled efficacy and safety trials were conducted in 641 subjects between the ages of 21 to 75 years, having Fitzpatrick Skin types I-IV and moderate to severe melasma of the face. TRI-LUMA Cream was compared with 3 possible combinations of 2 of the 3 active ingredients [(1) hydroquinone 4% (HQ) + tretinoin 0.05% (RA); (2) fluocinolone acetonide 0.01% (FA) + tretinoin 0.05% (RA); (3) fluocinolone acetonide 0.01% (FA) + hydroquinone 4% (HQ)], contained in the same vehicle as TRI-LUMA Cream. Subjects were instructed to apply their study medication each night, after washing their face with a mild soapless cleanser, for 8 weeks. Instructions were given to apply a thin layer of study medication to the hyperpigmented lesion, making sure to cover the entire lesion including the outside borders extending to the normal pigmented skin. Subjects were provided a mild moisturizer for use as needed. A sunscreen with SPF 30 was also provided with instructions for daily use. Protective clothing and avoidance of sunlight exposure to the face was recommended.
Subjects were evaluated for melasma severity at Baseline and at Weeks 1, 2, 4, and 8 of treatment. Primary efficacy was based on the proportion of subjects who had an investigators’ assessment of treatment success, defined as the clearing of melasma at the end of the eight-week treatment period. The majority of subjects enrolled in the two trials were white (approximately 66%) and female (approximately 98%). TRI-LUMA Cream was demonstrated to be significantly more effective than any of the other combinations of the active ingredients.
PRIMARY EFFICACY ANALYSIS:
Table 2. Investigators' Assessment of Treatment Success * At the End of 8 Weeks of Treatment - * Treatment success was defined as melasma severity score of zero (melasma lesions cleared of hyperpigmentation)
TRI-LUMA HQ+RA FA+RA FA+HQ Trial 1 Subjects, n 85 83 85 85 Successes, n 32 12 0 3 Proportion of Successes 38% 15% 0 4% p-value ‹ 0.001 ‹ 0.001 ‹ 0.001 Trial 2 Subjects, n 76 75 76 76 Successes, n 10 3 3 1 Proportion of Successes 13% 4% 4% 1% p-value 0.045 0.042 0.005 p-value is from Cochran-Mantel-Haenszel chi-square statistics controlling for pooled investigator and comparing TRI-LUMA Cream to the other treatment groups.
In the Investigators’ assessment of melasma severity at Day 56 of treatment, the following table shows the clinical improvement profile for all subjects treated with TRI-LUMA Cream based on severity of their melasma at the start of treatment.
Table 3. Investigators' Assessment of Change in Melasma Severity from Baseline to Day 56 of Treatment (combined results from trials 1 and 2) - * Assessment based on subjects with severity scores at Day 56. Percentages are bases on the total number in the treatment group population.
- † Does not include subjects who cleared before Day 56 or were missing from the Day 56 Assessment
Number (%) of Subjects at Day 56* Baseline Cleared † Mild Moderate Severe Missing Severity Rating n n (%) n (%) n (%) n (%) n (%) TRI-LUMA Cream N=161 Moderate 124 36 (29) 63 (51) 18 (15) 0 (0) 7 (6) Severe 37 6 (16) 19 (51) 9 (24) 2 (5) 1 (3) Assessment Scale: Cleared (melasma lesions approximately equivalent to surrounding normal skin or with minimal residual hyperpigmentation); Mild (slightly darker than the surrounding normal skin); Moderate (moderately darker than the surrounding normal skin); Severe (markedly darker than the surrounding normal skin).
Subjects experienced improvement of their melasma with the use of TRI-LUMA Cream as early as 4 weeks. However, among 7 subjects who had clearing at the end of 4 weeks of treatment with TRI-LUMA Cream, 4 of them did not maintain the remission after an additional 4 weeks of treatment.
After 8 weeks of treatment with the trial drug, subjects entered into an open-label extension period in which TRI-LUMA Cream was given on an as-needed basis for the treatment of melasma. The remission periods appeared to shorten between progressive courses of treatment. Additionally, few subjects maintained complete clearing of melasma (approximately 1 to 2%).
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
Inform patients of the following:
- Advise patients to change to non-hormonal forms of birth control, if hormonal methods are used.
- Use TRI-LUMA Cream as directed by the health care provider and do not use TRI-LUMA Cream for any disorder other than that for which it is prescribed.
- Avoid exposure to sunlight, sunlamp, or ultraviolet light. Patients who are consistently exposed to sunlight or skin irritants either through their work environment or habits should exercise particular caution. Use sunscreen and protective covering (such as the use of a hat) over the treated areas. Sunscreen use is an essential aspect of melasma therapy, as even minimal sunlight sustains melanocytic activity.
- Weather extremes, such as heat or cold, may be irritating to patients treated with TRI-LUMA Cream. Because of the drying effect of this medication, a moisturizer may be applied to the face in the morning after washing.
- Keep TRI-LUMA Cream away from the eyes, nose, angles of the mouth, or open wounds because these areas are more sensitive to the irritant effect. If local irritation persists or becomes severe, discontinue application of the medication and consult your health care provider. Seek medical attention if you experience allergic contact dermatitis, blistering, crusting, and severe burning or swelling of the skin and irritation of the mucous membranes of the eyes, nose, and mouth.
- If the medication is applied excessively, marked redness, peeling, or discomfort may occur.
- Wash your hands after each application.
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, TX 76177 USA
Manufactured by:Hill Dermaceuticals, Inc.
Sanford, FL 32773 USA
P51400-1
or
Manufactured by:
G Production Inc.
Baie d’Urfé, QC, H9X 3S4 Canada
Made in Canada
P52091-2
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
TRI-LUMA® (try-LOOM-ah)
(fluocinolone acetonide 0.01%, hydroquinone 4%, and tretinoin 0.05%)
Cream
Important information: TRI-LUMA Cream is for use on skin only. Do not use TRI-LUMA Cream in your mouth, eyes, or vagina.
What is the most important information I should know about TRI-LUMA Cream?
TRI-LUMA Cream may cause birth defects or death of the baby if used during pregnancy. The risk of birth defects or death of the baby may be greater if TRI-LUMA Cream is used during the first trimester of pregnancy. Tell your doctor if you are pregnant or plan to become pregnant.
If you become pregnant while using TRI-LUMA Cream, tell your doctor right away.
What is TRI-LUMA Cream?
TRI-LUMA Cream is a prescription medicine used for the short-term treatment of moderate to severe melasma of the face, in combination with sun avoidance and the use of sunscreens.
TRI-LUMA Cream is not for continuous treatment of melasma.
It is not known if TRI-LUMA Cream is safe and effective in children.
It is not known if TRI-LUMA Cream is safe and effective in people with dark brown to black skin color.
It is not known if TRI-LUMA Cream is safe and effective in the treatment of dark spots (hyperpigmentation) of the skin caused by conditions other than melasma of the face.
It is not known if TRI-LUMA Cream is safe and effective in females who are pregnant or who are breastfeeding. See "What is the most important information I should know about TRI-LUMA Cream? and What should I tell my doctor before using TRI-LUMA Cream?"
Who should not use TRI-LUMA Cream?
Do not use TRI-LUMA Cream if you are allergic to it or any of the ingredients in TRI-LUMA Cream. See the end of this leaflet for a complete list of ingredients in TRI-LUMA Cream.
What should I tell my doctor before using TRI-LUMA Cream?
Before you use TRI-LUMA Cream, tell your doctor if you:
- are allergic to sulfites
- have any other medical conditions
- are pregnant or plan to become pregnant. See "What is the most important information I should know about TRI-LUMA Cream?"
- are breastfeeding or plan to breastfeed. It is not known if TRI-LUMA Cream passes into your breast milk. You should avoid skin-to-skin contact between areas treated with TRI-LUMA Cream and your baby.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements and skin products that you use.
How should I use TRI-LUMA Cream?
- Use TRI-LUMA Cream exactly as your doctor tells you to use it.
- Before you apply TRI-LUMA Cream, gently wash your face with a mild cleanser. Rinse your face and pat your skin dry.
- Apply TRI-LUMA Cream 1 time a day, at least 30 minutes before bedtime.
- Apply a thin layer of TRI-LUMA Cream to the affected skin areas. Include about 1/2 inch of normal skin surrounding the affected area.
- Gently rub TRI-LUMA Cream evenly into your skin.
- Do not get TRI-LUMA Cream near the corners of your mouth, your nose, your eyes, or open wounds.
- Do not bandage or cover the treated skin after applying TRI-LUMA Cream.
- You may use moisturizers and cosmetics during the day.
- Wash your hands after applying TRI-LUMA Cream.
What should I avoid while using TRI-LUMA Cream?
- You should avoid sunlight, sunlamps, tanning beds, and ultraviolet light during treatment with TRI-LUMA Cream.
- Use a sunscreen with SPF (sun protection factor) of 30 or more. If you have to be in the sunlight, wear a wide-brimmed hat or other protective clothing to cover the treated areas.
- Melasma can get worse with even a small amount of sunlight. You should continue to avoid sunlight, use sunscreen, and wear protective clothing after treatment with TRI-LUMA Cream.
- Use a sunscreen with SPF (sun protection factor) of 30 or more. If you have to be in the sunlight, wear a wide-brimmed hat or other protective clothing to cover the treated areas.
- Females should avoid the use of hormonal forms of birth control. Hormonal birth control methods can cause your melasma to become worse. Talk to your doctor about other birth control options.
- Heat and cold weather may irritate skin treated with TRI-LUMA. Talk with your doctor about ways to manage skin irritation.
What are the possible side effects of TRI-LUMA Cream?
TRI-LUMA Cream may cause serious side effects, including:
-
allergic reactions. TRI-LUMA Cream may cause allergic reactions that can be life threatening. Stop using TRI-LUMA Cream and call your doctor to get medical help right away if you get any of the following symptoms:
- swelling of your face, eyes, lips, tongue, or throat
- trouble breathing
- severe itching
- skin rash or hives
- change in skin color. One of the medicines in TRI-LUMA Cream can cause a blue-black darkening of your skin. Stop using TRI-LUMA Cream and tell your doctor if you develop a blue-black darkening of your skin.
TRI-LUMA Cream can pass through your skin. Too much TRI-LUMA Cream passing through your skin can cause your adrenal glands to stop working. Your doctor may do blood tests to check for adrenal gland problems.
-
skin irritation. Stop using TRI-LUMA Cream and call your doctor if you have:
- blistering or crusting of your skin
- severe burning
- swelling of your skin
- irritation of your eyes, nose, or mouth
The most common side effects of TRI-LUMA Cream include:
- redness
- peeling
- burning
- dryness
- itching
- acne
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of TRI-LUMA Cream. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Galderma Laboratories, L.P. at 1-866-735-4137.
How should I store TRI-LUMA Cream?
- Store TRI-LUMA Cream in a refrigerator, between 36°F to 46°F (2°C to 8 °C).
- Keep TRI-LUMA Cream tube tightly closed.
- Do not freeze TRI-LUMA Cream.
General information about the safe and effective use of TRI-LUMA Cream
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TRI-LUMA Cream for a condition for which it was not prescribed. Do not give TRI-LUMA Cream to other people, even if they have the same symptoms you have. It may harm them.
If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about TRI-LUMA Cream that is written for health professionals.
What are the ingredients in TRI-LUMA Cream?
Active ingredients: fluocinolone acetonide, hydroquinone, and tretinoin
Inactive ingredients: butylated hydroxytoluene, cetyl alcohol, citric acid anhydrous, glycerin, glyceryl stearate, magnesium aluminum silicate, methyl gluceth-10, methylparaben, PEG-100 stearate, propylparaben, purified water, sodium metabisulfite, stearic acid, and stearyl alcohol
This Patient Information has been approved by the U.S. Food and Drug Administration.
Marketed by:GALDERMA LABORATORIES, L.P.
Fort Worth, TX 76177 USA
Manufactured by:Hill Dermaceuticals, Inc.
Sanford, FL 32773 USA
or
Manufactured by:
G Production Inc.
Baie dUrfé, QC, H9X 3S4 Canada
Made in Canada
Revised: March 2014
-
PRINCIPAL DISPLAY PANEL

Tri-Luma®(fluocinolone acetonide, hydroquinone, tretinoin) cream, 0.01%/4%/0.05%
MUST BE REFRIGERATED
NDC 0299-5950-30Rx Only
NET WT. 30 g
GALDERMA
For Topical Use Only. Not for Ophthalmic Use.
Usual dosage: Apply a thin film to affected areas once daily at night. See package insert for complete prescribing information.
Each gram contains: Active: fluocinolone acetonide 0.01% (0.1 mg), hydroquinone 4% (40 mg), and tretinoin 0.05% (0.5 mg). Inactive: butylated hydroxytoluene, cetyl alcohol, citric acid anhydrous, glycerin, glyceryl stearate, magnesium aluminum silicate, methyl gluceth-10, methylparaben, PEG-100 stearate, propylparaben, purified water, sodium metabisulfite, stearic acid, and stearyl alcohol.
Storage: Store in a refrigerator, 2° to 8°C (36° to 46°F). Protect from freezing.
See carton closure for lot number and expiration date.
www.triluma.com
Marketed by:GALDERMA LABORATORIES, L.P.Fort Worth, Texas 76177 USA
Galderma is a registered trademark.
P51399-3
MUST BE REFRIGERATED -
INGREDIENTS AND APPEARANCE
TRI-LUMA
fluocinolone acetonide, hydroquinone, and tretinoin creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0299-5950 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOCINOLONE ACETONIDE (UNII: 0CD5FD6S2M) (FLUOCINOLONE ACETONIDE - UNII:0CD5FD6S2M) FLUOCINOLONE ACETONIDE 0.1 mg in 1 g HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 40 mg in 1 g TRETINOIN (UNII: 5688UTC01R) (TRETINOIN - UNII:5688UTC01R) TRETINOIN 0.5 mg in 1 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CETYL ALCOHOL (UNII: 936JST6JCN) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) METHYL GLUCETH-10 (UNII: N0MWT4C7WH) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-100 STEARATE (UNII: YD01N1999R) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM METABISULFITE (UNII: 4VON5FNS3C) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0299-5950-30 1 in 1 CARTON 01/18/2007 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0299-5950-02 1 in 1 BLISTER PACK 04/01/2012 2 3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021112 01/18/2002 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations Hill Dermaceuticals, Inc. 098366990 manufacture(0299-5950) Establishment Name Address ID/FEI Business Operations G Production, Inc. 251676961 manufacture(0299-5950)
Trademark Results [TRI-LUMA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRI-LUMA 75889914 2605369 Live/Registered |
NESTLÃ SKIN HEALTH S.A. 2000-01-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.