GALLIPRANT- grapiprant tablet
Galliprant by
Drug Labeling and Warnings
Galliprant by is a Animal medication manufactured, distributed, or labeled by Elanco US Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
(grapiprant tablets)
For oral use in dogs only
20 mg, 60 mg and 100 mg flavored tablets
A prostaglandin E2 (PGE2) EP4 receptor antagonist; a non-cyclooxygenase inhibiting, non-steroidal anti-inflammatory drug
Caution:
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
-
Description:
GALLIPRANT (grapiprant tablets) is a prostaglandin E2 (PGE2) EP4 receptor antagonist; a non-cyclooxygenase (COX) inhibiting, non-steroidal anti-inflammatory drug (NSAID) in the piprant class. GALLIPRANT is a flavored, oval, biconvex, beige to brown in color, scored tablet debossed with a “G” that contains grapiprant and desiccated pork liver as the flavoring agent.
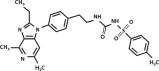
The molecular weight of grapiprant is 491.61 Daltons. The empirical formula is C26H29N5O3S. Grapiprant is N-[[[2-[4-(2-Ethyl-4,6-dimethyl-1H-imidazo[4,5-c] pyridin-1-yl)phenyl]ethyl]amino]carbonyl]-4 methylbenzenesulfonamide.
The structural formula is:
- Indication:
-
Dosage and Administration:
Always provide “Information for Dog Owners” Sheet with prescription. Use the lowest effective dose for the shortest duration consistent with individual response.
The dose of GALLIPRANT (grapiprant tablets) is 0.9 mg/lb (2 mg/kg) once daily. Only the 20 mg and 60 mg tablets of GALLIPRANT are scored.
The dosage should be calculated in half tablet increments. Dogs less than 8 lbs. (3.6 kgs) cannot be accurately dosed.
Dosing Chart
Dose Weight in pounds Weight in kilograms 20 mg tablet 60 mg tablet 100 mg tablet
0.9 mg/lb (2 mg/kg) once daily8-15 3.6-6.8 0.5 15.1-30 6.9-13.6 1 30.1-45 13.7-20.4 0.5 45.1-75 20.5-34 1 75.1-150 34.1-68 1 The 100 mg tablet is not scored and should not be broken in half.
Breaking the 100 mg tablet in half will not guarantee that half of the active ingredient is contained within each half of the tablet. For dogs larger than 150 lbs (68 kgs), use a combination of tablet and half tablets to achieve the appropriate dose.
- Contraindications:
-
Warnings:
Not for use in humans. Keep this and all medications out of reach of children and pets. Consult a physician in case of accidental ingestion by humans.
For use in dogs only. Store GALLIPRANT out of reach of dogs and other pets in a secured location in order to prevent accidental ingestion or overdose.
-
Precautions:
The safe use of GALLIPRANT has not been evaluated in dogs younger than 9 months of age and less than 8 lbs (3.6 kg), dogs used for breeding, or in pregnant or lactating dogs.
Adverse reactions in dogs receiving GALLIPRANT may include vomiting, diarrhea, decreased appetite, mucoid, watery or bloody stools, and decreases in serum albumin and total protein.
If GALLIPRANT is used long term appropriate monitoring is recommended.
Concurrent use with other anti-inflammatory drugs has not been studied. Concomitant use of GALLIPRANT with other anti-inflammatory drugs, such as COX-inhibiting NSAIDs or corticosteroids, should be avoided. If additional pain medication is needed after a daily dose of GALLIPRANT, a non-NSAID/non-corticosteroid class of analgesic may be necessary.
The concomitant use of protein-bound drugs with GALLIPRANT has not been studied. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications.
Drug compatibility should be monitored in patients requiring adjunctive therapy. Consider appropriate washout times when switching from one anti-inflammatory to another or when switching from corticosteroids or COX-inhibiting NSAIDs to GALLIPRANT use.
The use of GALLIPRANT in dogs with cardiac disease has not been studied.
It is not known whether dogs with a history of hypersensitivity to sulfonamide drugs will exhibit hypersensitivity to GALLIPRANT. GALLIPRANT is a methylbenzenesulfonamide.
-
Adverse Reactions:
In a controlled field study, 285 dogs were evaluated for safety when given either GALLIPRANT or a vehicle control (tablet minus grapiprant) at a dose of 2 mg/kg (0.9 mg/lb) once daily for 28 days. GALLIPRANT-treated dogs ranged in age from 2 yrs to 16.75 years. The following adverse reactions were observed:
Table 1. Adverse reactions reported in the field study. *Dogs may have experienced more than one type or occurrence during the study.
Adverse reaction*GALLIPRANT
(grapiprant tablets) N = 141Vehicle control (tablets minus grapiprant)
N = 144Vomiting 24 9 Diarrhea, soft stool 17 13 Anorexia, inappetence 9 7 Lethargy 6 2 Buccal ulcer 1 0 Immune mediated hemolytic anemia 1 0 GALLIPRANT was used safely during the field studies with other concurrent therapies, including antibiotics, parasiticides and vaccinations.
To report suspected adverse drug events and/or to obtain a copy of the Safety Data Sheet (SDS) or for technical assistance, call 1-888-545-5973.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth
-
Information for Dog Owners:
Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include vomiting, diarrhea, decreased appetite, and decreasing albumin and total protein.
Appetite and stools should be monitored and owners should be advised to consult with their veterinarian if appetite decreases or stools become abnormal.
-
Clinical Pharmacology:
Grapiprant is a prostaglandin E2 (PGE2) EP4 receptor antagonist; a non- cyclooxygenase inhibiting, non-steroidal, anti-inflammatory drug. Grapiprant has a canine EP4 receptor binding affnity (Ki) of 24 nM.
Prostaglandins have a wide variety of physiologic effects. Prostaglandin E2 (PGE2) is a prostanoid that exerts its effects via four receptors, EP1, EP2, EP3, and EP4. PGE2 is involved in mediating inflammatory pain, vasodilation, increasing vascular permeability; as well as gastrointestinal homeostasis, renal function and reproductive functions. The EP4 receptor is important in mediating pain and inflammation as it is the primary mediator of the PGE2-elicited sensitization of sensory neurons1 and PGE2-elicited inflammation.2 Grapiprant blocks PGE2-elicited pain and inflammation by antagonizing the EP4 receptor.
The EP4 receptor, along with the EP1, EP2 and EP3 receptors, is involved in PGE2 mediated effects on gastrointestinal homeostasis and renal function. PGE2 effects mediated solely by the EP4 receptor are stimulation of mucus secretion in the stomach and large intestine, stimulation of acid secretion in the stomach, inhibition of small intestine motility and inhibition of cytokine expression in the large intestine.3 While PGE2 gastroprotective action is mediated by EP1, the healing- promoting action of PGE2 in the stomach is mediated by the EP4 receptor.4 In the kidney, the PGE2 antinatiuretic effect is mediated by the EP4 receptor.5
EP4 receptors are abundantly expressed in the heart of dogs,6 the clinical relevance of which is unknown. The EP4 receptor is not involved in generation of pyrexia.
Grapiprant is not a potential inhibitor of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 mediated metabolism pathways. Grapiprant is a substrate of P-glycoprotein transport. In vitro metabolism with dog liver microsomes identified two oxidative metabolites, M3 (hydroxyl) and M5 (N-dealkylation).
The pharmacokinetic characterization of grapiprant following oral administration of GALLIPRANT tablets to healthy Beagles is provided in the table below.
Table 2. Mean (±SD) Plasma Pharmacokinetic Parameters for Grapiprant in Beagles after single oral dose of GALLIPRANT tablet formulation 1Study 1 was a food effect determination study.
2Study 2 was a PK bridging study conducted using 60 mg GALLIPRANT tablets at 6 mg/kg dose and 5 X 100 mg GALLIPRANT tablets at 50 mg/kg dose.
3Median (Range)
Study Study 11 Study 11 Study 22 Study 22
PK Parameter2 mg/kg
(n = 10)
(Fasted)2 mg/kg
(n = 10)
(Fed)6 mg/kg
(n = 8)
(Fasted)50 mg/kg
(n = 8)
(Fasted)Tmax3 (hr) 1.0
(0.5 – 1.03)1.0
(0.5 – 8.07)1.0
(1.0 – 2.0)2.0
(1.0 – 4.0)Cmax (ng/mL) 1210
(341)278
(179)5720
(3220)98500
(13100)AUC(0-inf) (ng*hr/mL) 2790
(982)1200
(523)17800
(5520)414000
(73700)T1/2
(hr)4.60
(4.19)5.67
(3.27)5.01
(1.95)5.21
(1.66)Fed/Fasted Relative Bioavailability Geometric Mean Ratio of AUC
(90% Confidence Limits)
0.37 (0.28 – 0.46)
NAGrapiprant is absorbed rapidly following an oral dose of the GALLIPRANT; with Cmax values achieved within approximately 2 hr post-dose (Tmax). Intake of the tablet with food significantly reduces the oral bioavailability, with mean Cmax and AUC grapiprant values reduced 4-fold and 2-fold, respectively. The systemic grapiprant exposure increases in a greater than dose proportional manner.
The mean terminal elimination half-life (T1/2) ranges between 4.60 to 5.67 hr. Following once daily dosing, negligible drug accumulation in the blood is anticipated. Following an oral dose of radiolabeled grapiprant to dogs, the majority of the dose was excreted within the first 72 hr (84%) and approximately 88.7% of the dose was excreted in 192 hr. In a bile duct cannulated dog study, approximately 55.6%, 15.1% and 19.1% of the dose was excreted in bile, urine and feces, respectively, suggesting the high oral bioavailability of grapiprant in dogs (> 70%). Four metabolites were identified; two hydroxylated metabolites, one N-deamination metabolite (major metabolite urine (3.4%) and feces (7.2%)) and one N-oxidation metabolite. Metabolite activity is not known. Plasma protein binding of grapiprant was ~95%.
-
Effectiveness:
Two hundred and eighty five (285) client-owned dogs were enrolled in the study and evaluated for field safety. GALLIPRANT-treated dogs ranging in age from 2 to 16.75 years and weighing between 4.1 and 59.6 kgs (9 – 131 lbs) with radiographic and clinical signs of osteoarthritis were enrolled in a placebo-controlled, masked field study. Dogs had a 7-day washout from NSAID or other current OA therapy. Two hundred and sixty two (262) of the 285 dogs were included in the effectiveness evaluation. Dogs were assessed for improvements in pain and function by the owners using the Canine Brief Pain Inventory (CBPI) scoring system.7 A statistically significant difference in the proportion of treatment successes in the GALLIPRANT group (63/131 or 48.1%) was observed compared to the vehicle control group (41/131 or 31.3%). GALLIPRANT demonstrated statistically significant differences in owner assessed pain and function. The results of the field study demonstrate that GALLIPRANT, administered at 2 mg/kg (0.9 mg/pound) once daily for 28 days, was effective for the control of pain and inflammation associated with osteoarthritis.
-
Animal Safety:
In a 9-month toxicity study, grapiprant in a methylcellulose suspension was administered by oral gavage once daily to healthy Beagles at doses of 1, 6, and 50 mg/kg/day. Based on a relative bioavailability study comparing grapiprant in methylcellulose suspension to GALLIPRANT tablets, the corresponding equivalent doses were 0.75 mg/kg (0.12X – 0.25X), 4.44 mg/kg (0.72X – 1.48X) and 30.47 mg/kg (4.88X – 10.16X) of the GALLIPRANT tablets. Four animals/sex were used in each dose group and 2 additional animals/sex were used in the 50 mg/kg dose group to evaluate recovery after drug cessation. Vomiting and soft-formed or mucus stool were observed in all groups, including controls, with higher incidence in grapiprant-treated dogs. Decreases in serum albumin and total protein were seen with increasing doses of grapiprant. Hypoalbuminemia and hypoproteinemia were reversible when treatment was discontinued. Three treated dogs and one control dog had elevated alkaline phosphatase values. One animal in the 50 mg/kg group (equivalent to 30.47 mg/kg of tablet formulation) had mild regeneration of the mucosal epithelium of the ileum.
In a field study conducted in 366 client-owned dogs to evaluate GALLIPRANT at doses of 2 mg/kg once daily, 5 mg/kg once daily, 4 mg/kg twice daily, or placebo twice daily, the most common adverse reactions related to treatment were diarrhea, vomiting and inappetence. Changes in clinical pathology included concurrent elevations of alkaline phosphatase and alanine aminotransferase values on Day 28, and dose-dependent decreases in total protein values. There was no clinical impact related to these clinical pathology changes.
- Storage Conditions:
- How Supplied:
-
References:
- Nakao, K., Murase, A., et al. CJ-023,423, a novel, potent and selective prostaglandin EP4 receptor antagonist with antihyperalgesic properties. The Journal of Pharmacology and Experimental Therapeutics. 2007; 322(2), 686-694.
- Murase, A., Okumura, T., et al. Effect of prostanoid EP4 receptor antagonist, CJ-042,794, in rat models of pain and inflammation. European Journal of Pharmacology. 2008; 580(1-2), 116-121.
- Takeuchi, K., S. Kato, et al. Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. Journal of Pharmacological Sciences. 2010; 114(3): 248-261.
- Hatazawa R, Tanaka A, Tanigami M, et al. Cyclooxygenase-2/prostaglandin E2 accelerates the healing of gastric ulcers via EP4 receptors. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007; 293: G788-G797.
- Nasrallah R, Hassouneh R, and Hebert R. Chronic kidney disease: targeting prostaglandin E2 receptors. American Journal of Physiology Renal Physiology. 2014; 307: F242-250.
- Castleberry TA, Lu B, et al. Molecular cloning and functional characterization of the canine prostaglandin E2 receptor EP4 subtype. Prostaglandins and Other Lipid Mediators. 2001; 65: 167-187.
- http://www.vet.upenn.edu/docs/default-source/ VCIC/canine-bpi_userguide. pdf?sfvrsn=0
Galliprant is the registered trademark of Aratana Therapeutics, Inc. Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
February 2018
PA101390XARATANA
THERAPEUTICS®ElancoTM
Galliprant®
(grapiprant tablets) -
INFORMATION FOR OWNERS/CAREGIVERS
For oral use in dogs only
Flavored tablets
A prostaglandin E2 (PGE2) EP4 receptor antagonist; a non-cyclooxygenase inhibiting, non-steroidal, anti-inflammatory drug.
Information for Dog Owners
GALLIPRANT is indicated for the control of pain and inflammation due to osteoarthritis.
This sheet contains important information about GALLIPRANT.
You should read this information before starting your dog on GALLIPRANT and review it each time the prescription is refilled. This information provides a summary and does not take the place of the instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or you want more information about GALLIPRANT.
GALLIPRANT is a prostaglandin E2 (PGE2) EP4 receptor antagonist; a non-cyclooxygenase (COX) inhibiting, non-steroidal, anti-inflammatory drug (NSAID). As an anti-inflammatory, GALLIPRANT is indicated for the control of pain and inflammation associated with osteoarthritis in dogs.
Control of pain and inflammation may vary from dog to dog. Consult your veterinarian if your dog appears to be uncomfortable. GALLIPRANT may need to be given for an extended period of time. Use the lowest dose to provide adequate pain control. Always consult with your veterinarian before altering the dose.
It is important to periodically discuss your dog's response to GALLIPRANT with your veterinarian. Your veterinarian will discuss appropriate monitoring while your dog is on GALLIPRANT.
The most common side effects associated with GALLIPRANT include vomiting, soft, mucoid stools, diarrhea and decreased appetite. You should contact your veterinarian if your dog's appetite decreases or stools become abnormal.
Dogs should not take GALLIPRANT if:
- Your dog is presently taking aspirin, other NSAIDs, or corticosteroids (unless directed by your veterinarian).
- Your dog has had an allergic reaction (such as hives, facial swelling, or itchy skin) to GALLIPRANT. Inform your veterinarian if your dog has had an allergic reaction to other anti-inflammatory drugs.
- Your dog is under 8 pounds in body weight.
GALLIPRANT should only be given to dogs. Do not use in cats.
GALLIPRANT should not be given with other non-steroidal anti-inflammatory drugs (NSAIDs) or corticosteroids (for example, aspirin, carprofen, meloxicam and prednisone).
Not for use in humans. Keep this and all medications out of reach of children and pets. Consult a physician in case of accidental ingestion by humans.
Store GALLIPRANT out of reach of dogs and other pets in a secured location in order to prevent accidental ingestion or overdose.
Tell your veterinarian about:
- Any side effects your dog has experienced from GALLIPRANT or other NSAIDs.
- Any digestive issues (bloody, mucoid stool, vomiting or diarrhea, or decreased appetite) your dog has.
- Any other medical problems or allergies that your dog has had.
- All medications that you are giving your dog or plan to give your dog, including those you can get without a prescription and any dietary supplements.
- If you plan to breed your dog, or if your dog is pregnant or nursing.
If you have additional questions about possible side effects while your dog is on GALLIPRANT, talk with your veterinarian or call 1-888-545-5973.
Galliprant is the registered trademark of Aratana Therapeutics, Inc.
Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
February 2018
ARATANA
THERAPEUTICS®PA101390X
ElancoTM
-
Principal Display Panel - Galliprant 20 mg 7 Tablets Box Label
GALLIPRANT®(grapiprant tablets)
20 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
7 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844A
- Principal Display Panel - Galliprant 20 mg 7 Tablets Carton Label
- Principal Display Panel - Galliprant 20 mg 7 Tablets Bottle Label
-
Principal Display Panel - Galliprant 20 mg 30 Tablets Box Label
GALLIPRANT®(grapiprant tablets)
20 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
30 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844B
- Principal Display Panel - Galliprant 20 mg 30 Tablets Carton Label
- Principal Display Panel - Galliprant 20 mg 30 Tablets Bottle Label
-
Principal Display Panel - Galliprant 20 mg 90 Tablets Box Label
GALLIPRANT®(grapiprant tablets)
20 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
90 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844B
- Principal Display Panel - Galliprant 20 mg 90 Tablets Carton Label
- Principal Display Panel - Galliprant 20 mg 90 Tablets Bottle Label
-
Principal Display Panel - Galliprant 60 mg 7 Tablets Box Label
GALLIPRANT®(grapiprant tablets)
60 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
7 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844D
- Principal Display Panel - Galliprant 60 mg 7 Tablets Carton Label
- Principal Display Panel - Galliprant 60 mg 7 Tablets Bottle Label
-
Principal Display Panel - Galliprant 60 mg 30 Tablets Box Label
GALLIPRANT®(grapiprant tablets)
60 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
30 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844E
- Principal Display Panel - Galliprant 60 mg 30 Tablets Carton Label
- Principal Display Panel - Galliprant 60 mg 30 Tablets Bottle Label
-
Principal Display Panel - Galliprant 20 mg 90 Tablets Box Label
GALLIPRANT® (grapiprant tablets)
60 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
90 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844F
- Principal Display Panel - Galliprant 60 mg 90 Tablets Carton Label
- Principal Display Panel - Galliprant 60 mg 90 Tablets Bottle Label
-
Principal Display Panel - Galliprant 100 mg 7 Tablets Box Label
GALLIPRANT® (grapiprant tablets)
100 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
7 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844G
- Principal Display Panel - Galliprant 100 mg 7 Tablets Carton Label
- Principal Display Panel - Galliprant 100 mg 7 Tablets Bottle Label
-
Principal Display Panel - Galliprant 100 mg 30 Tablets Box Label
GALLIPRANT® (grapiprant tablets)
100 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
30 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844H
- Principal Display Panel - Galliprant 100 mg 30 Tablets Carton Label
- Principal Display Panel - Galliprant 100 mg 30 Tablets Bottle Label
-
Principal Display Panel - Galliprant 20 mg 90 Tablets Box Label
GALLIPRANT® (grapiprant tablets)
100 mg Flavored Tablets
Store at or below 86° F (30° C)
Caution: Federal (USA)
law restricts this drug to
use by or on the order of
a licensed veterinarian.For oral use in dogs only
Case Qty: 48 (1 bottle) cartons
90 flavored tablets per bottle
NADA 141-455
Approved by FDA
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140
1-888-545-5973
YL100844I
- Principal Display Panel - Galliprant 100 mg 90 Tablets Carton Label
- Principal Display Panel - Galliprant 100 mg 90 Tablets Bottle Label
-
INGREDIENTS AND APPEARANCE
GALLIPRANT
grapiprant tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 58198-5810 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Grapiprant (UNII: J9F5ZPH7NB) (Grapiprant - UNII:J9F5ZPH7NB) Grapiprant 20 mg Product Characteristics Color brown (Brown) Score 2 pieces Shape OVAL (OVAL) Size 12mm Flavor Imprint Code 20;mg;G;G Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-5810-4 48 in 1 BOX 1 NDC: 58198-5810-1 1 in 1 CARTON 1 7 in 1 BOTTLE 2 NDC: 58198-5810-5 48 in 1 BOX 2 NDC: 58198-5810-2 1 in 1 CARTON 2 30 in 1 BOTTLE 3 NDC: 58198-5810-6 48 in 1 BOX 3 NDC: 58198-5810-3 1 in 1 CARTON 3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141455 01/03/2016 GALLIPRANT
grapiprant tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 58198-5811 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Grapiprant (UNII: J9F5ZPH7NB) (Grapiprant - UNII:J9F5ZPH7NB) Grapiprant 60 mg Product Characteristics Color brown (Brown) Score 2 pieces Shape OVAL (OVAL) Size 18mm Flavor Imprint Code 60;mg;G;G Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-5811-4 48 in 1 BOX 1 NDC: 58198-5811-1 1 in 1 CARTON 1 7 in 1 BOTTLE 2 NDC: 58198-5811-5 48 in 1 BOX 2 NDC: 58198-5811-2 1 in 1 CARTON 2 30 in 1 BOTTLE 3 NDC: 58198-5811-6 48 in 1 BOX 3 NDC: 58198-5811-3 1 in 1 CARTON 3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141455 01/03/2016 GALLIPRANT
grapiprant tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 58198-5812 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Grapiprant (UNII: J9F5ZPH7NB) (Grapiprant - UNII:J9F5ZPH7NB) Grapiprant 100 mg Product Characteristics Color brown (Brown) Score no score Shape OVAL (OVAL) Size 23mm Flavor Imprint Code 100;mg;G;G Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-5812-4 48 in 1 BOX 1 NDC: 58198-5812-1 1 in 1 CARTON 1 7 in 1 BOTTLE 2 NDC: 58198-5812-5 48 in 1 BOX 2 NDC: 58198-5812-2 1 in 1 CARTON 2 30 in 1 BOTTLE 3 NDC: 58198-5812-6 48 in 1 BOX 3 NDC: 58198-5812-3 1 in 1 CARTON 3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141455 01/03/2016 Labeler - Elanco US Inc. (966985624)
Trademark Results [Galliprant]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GALLIPRANT 86977764 4887025 Live/Registered |
ARATANA THERAPEUTICS, INC. 2014-03-28 |
 GALLIPRANT 86977763 4883237 Live/Registered |
ARATANA THERAPEUTICS, INC. 2014-10-08 |
 GALLIPRANT 86975749 4745253 Live/Registered |
ARATANA THERAPEUTICS, INC. 2014-03-28 |
 GALLIPRANT 86788230 4964554 Live/Registered |
ARATANA THERAPEUTICS, INC. 2015-10-14 |
 GALLIPRANT 86417918 not registered Dead/Abandoned |
ARATANA THERAPEUTICS, INC. 2014-10-08 |
 GALLIPRANT 86235377 not registered Dead/Abandoned |
ARATANA THERAPEUTICS, INC. 2014-03-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.