EXTRA STRENGTH PAIN AID- acetaminophen, aspirin, caffeine tablet
Extra Strength Pain Aid by
Drug Labeling and Warnings
Extra Strength Pain Aid by is a Otc medication manufactured, distributed, or labeled by Hi-Tech Nutaceuticals, LLC, Hi-Tech Nutraceuticals, LLC, Hi-Tech Nuraceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- QUESTIONS
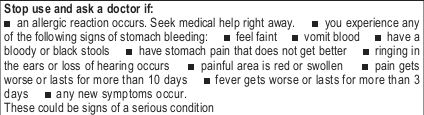
- STOP USE
- WHEN USING
- WARNINGS
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- STORAGE AND HANDLING
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EXTRA STRENGTH PAIN AID
acetaminophen, aspirin, caffeine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69732-006 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 250 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score score with uneven pieces Shape ROUND Size 10mm Flavor Imprint Code HT565 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69732-006-01 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/30/2020 Labeler - Hi-Tech Nutaceuticals, LLC (606221443) Establishment Name Address ID/FEI Business Operations Hi-Tech Nutraceuticals, LLC 080787135 pack(69732-006) Establishment Name Address ID/FEI Business Operations Hi-Tech Nuraceuticals, LLC 606221443 manufacture(69732-006)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.