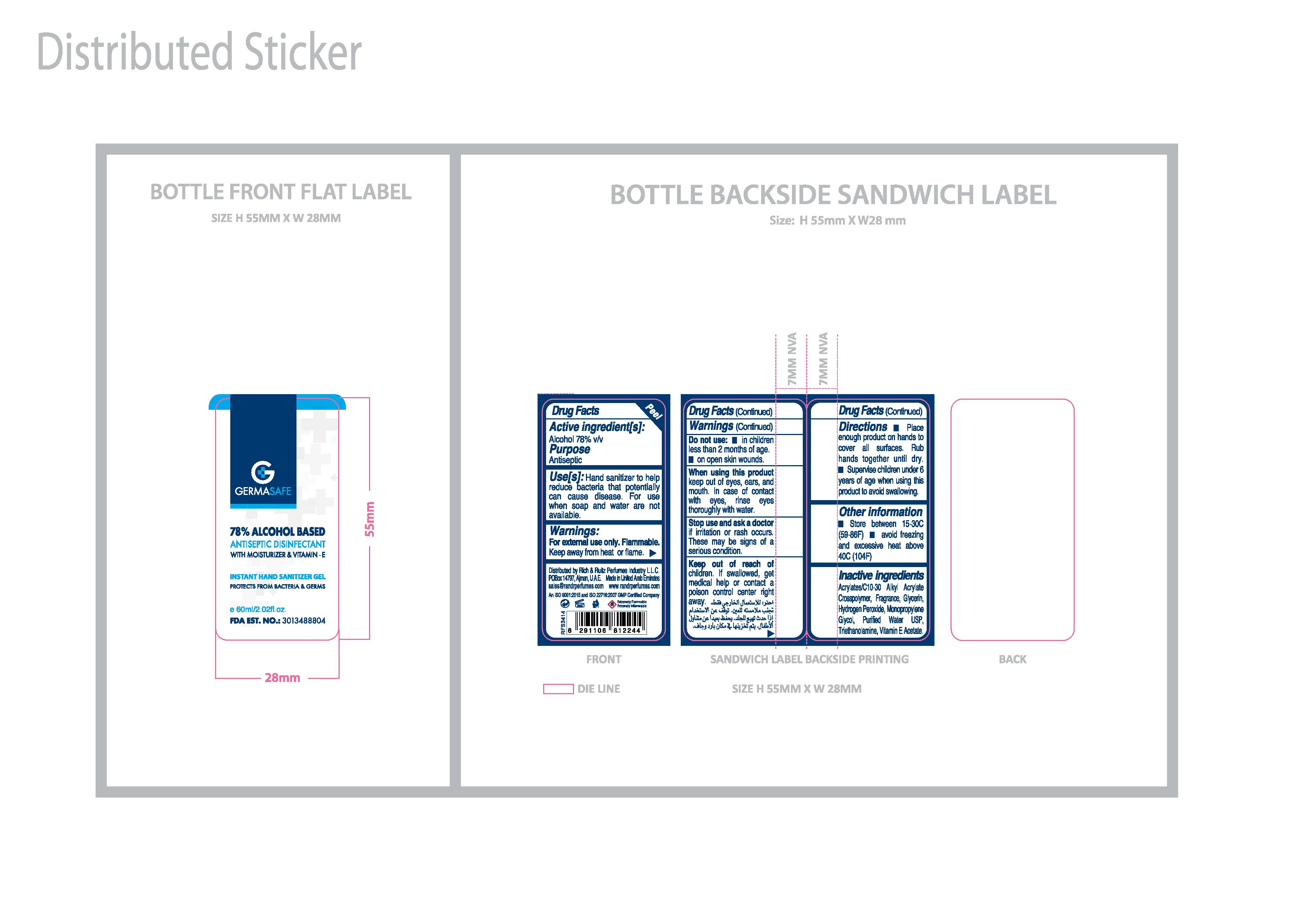
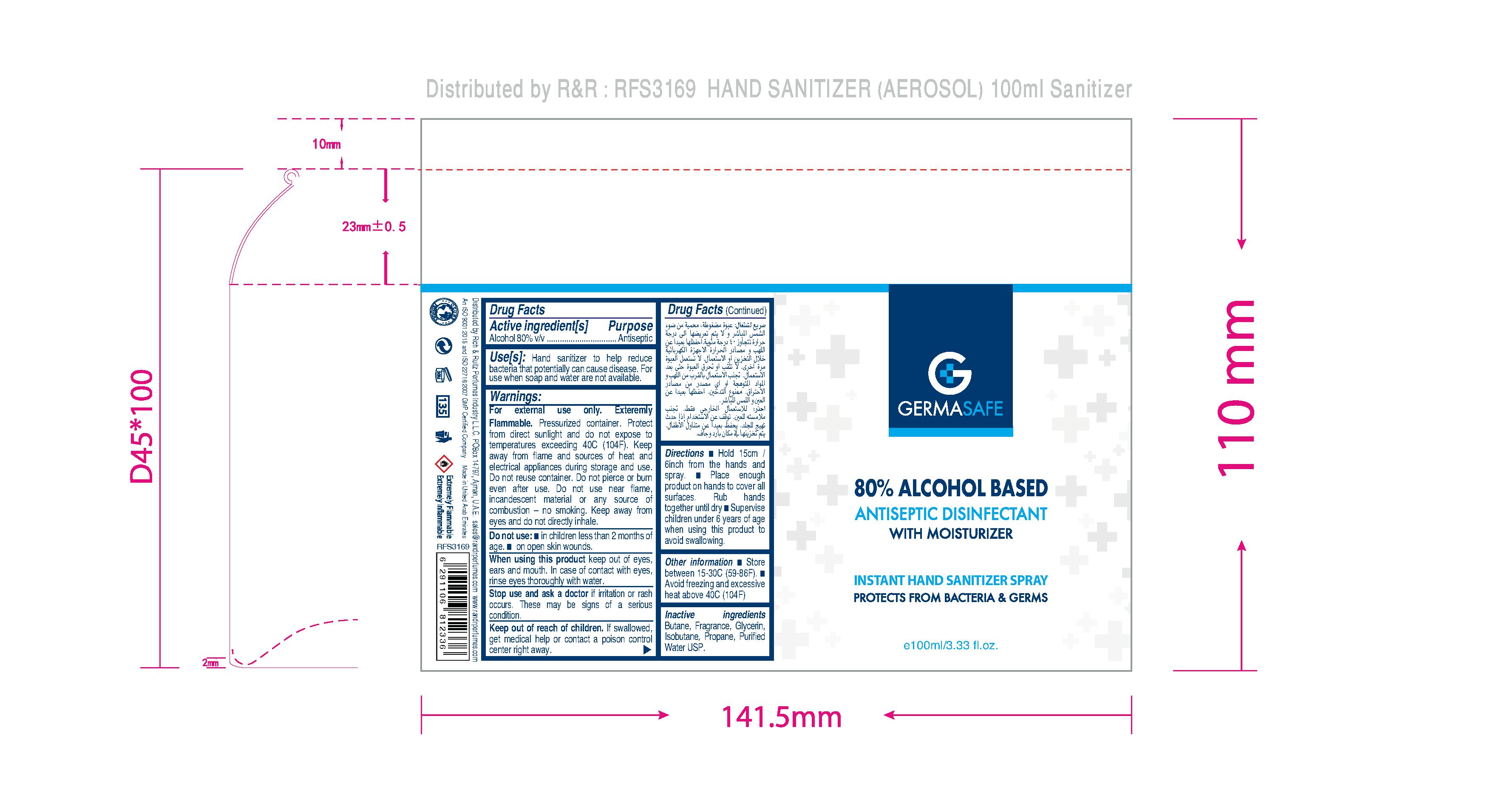
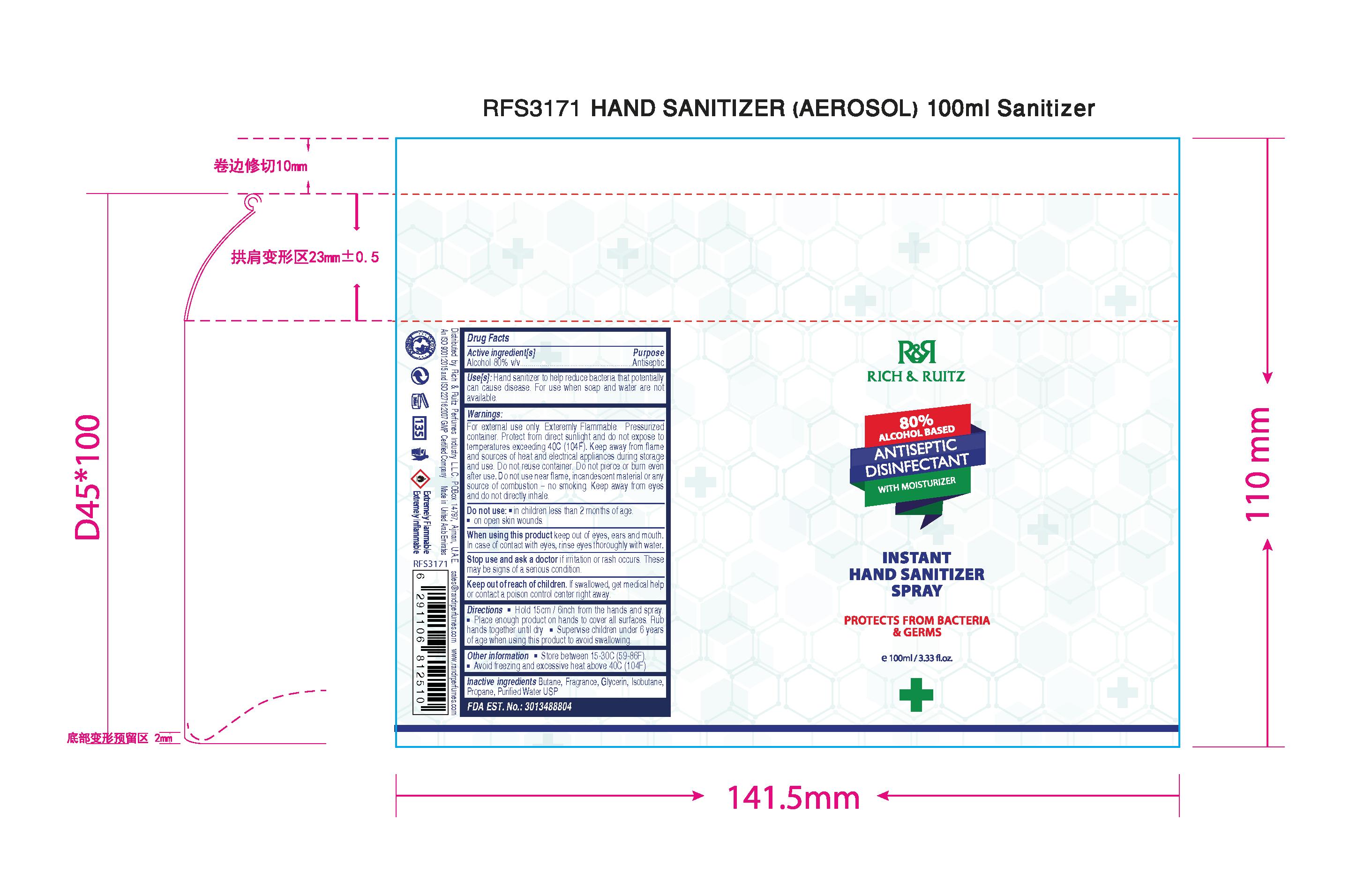
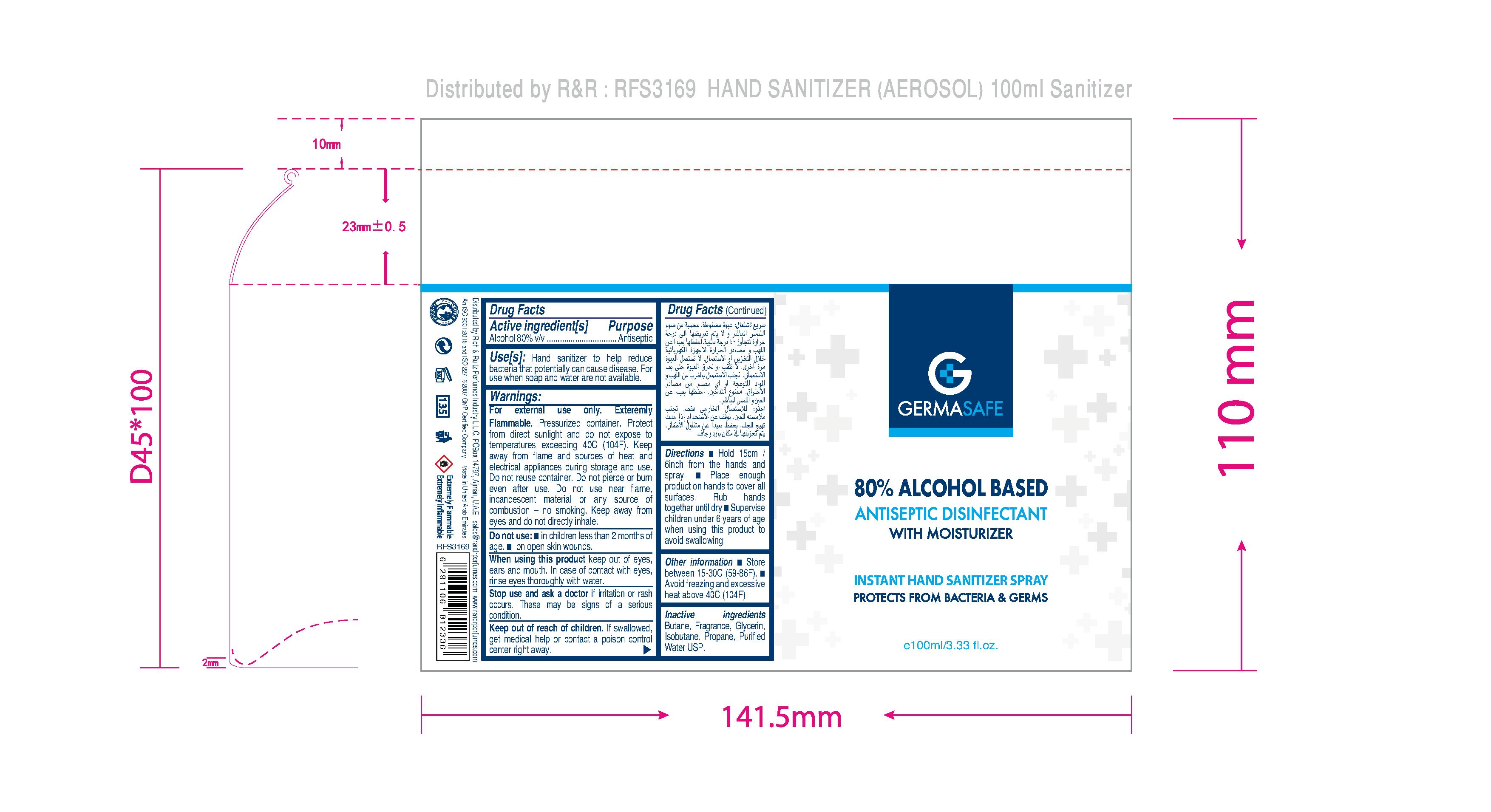
HAND SANITIZER





GERMASAFE HAND SANITIZER GEL
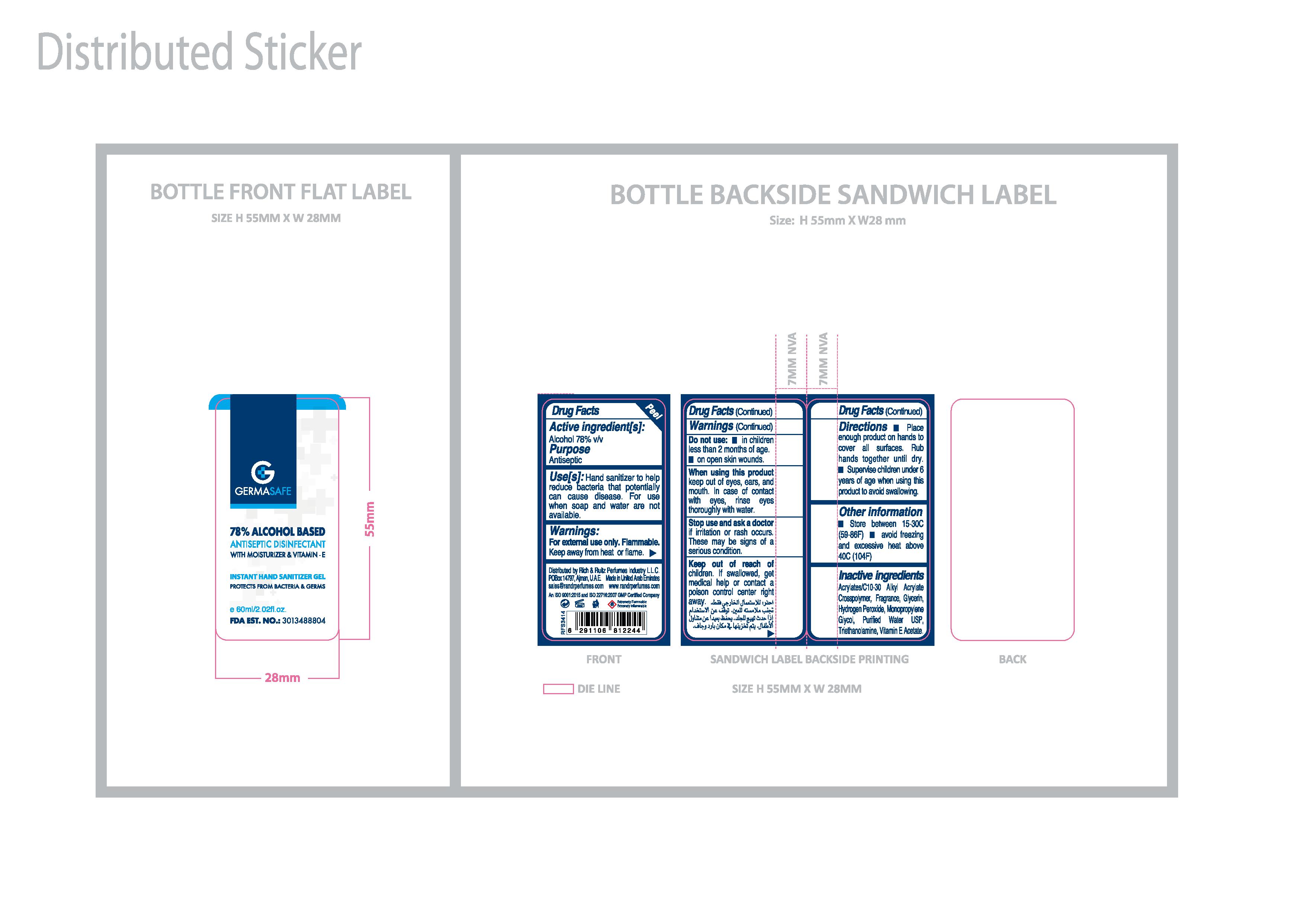
Germasafe Gel's Active Ingredient is Alcohol 78% v/v
GERMASAFE HAND SANITIZER SPRAY
Alcohol 80% v/v
HAND SANITIZERS
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition. Contained in labels for Germasafe Gel (60ml, 200ml, 250ml, 500ml), Germasafe Spray (100ml) and R&R Spray (100ml)
HAND SANITIZER
Contained in all Germasafe and R&R products:
Do not use:
- in children less than 2 months of age.
- on open skin wounds.
HAND SANITIZER
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away. Contained in all Germasafe and R&R products.
HAND SANITIZER
Purpose: Antiseptic
Contained in all Germasafe and R&R products.
HAND SANITIZER
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition. Contained in labels of all Germasafe and R&R products.
HAND SANITIZER
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Contained on labels of all Germasafe and R&R products.
HAND SANITIZER, GEL
Directions:
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
HAND SANITIZER, SPRAY
Directions:
- Hold 15cm/6inch from the hands and spray.
- Place enough prouct on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
HAND SANITIZER
Inactive ingredients: Butane, Fragrance, Glycerin, Isobutane, Propane, Purified Water USP.
HAND SANITIZER
Use[s]: Hand sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
HAND SANITIZER GEL
Directions:
Place enough product on hands to cover all surfaces. Rub hands together until dry.
Supervise children under 6 years of age when using this product to avoid swallowing.
HAND SANITIZER SPRAY
Directions:
Hold 15cm/6inch from the hands and spray.
Place enough prouct on hands to cover all surfaces. Rub hands together until dry.
Supervise children under 6 years of age when using this product to avoid swallowing.
HAND SANITIZER
Other Information:
- Store between 15-30C (59-86F)
- Avoid freezing and excessive heat above 40C (104F)
HAND SANITIZER
Warnings:
For external use only. Extremely Flammable. Pressurized container. Protect from direct sunlight and do not expose to temperatures exceeding 40C (104F). Keep away from flame and sources of heat and electrical appliances during storage and use. Do not use near flame, incandescent material or any source of combustion - no smoking. Keep away from eyes and do not directly inhale.