SULFACETAMIDE SODIUM solution/ drops
Sulfacetamide Sodium by
Drug Labeling and Warnings
Sulfacetamide Sodium by is a Prescription medication manufactured, distributed, or labeled by Akorn, Inc., Akorn, Inc. . Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
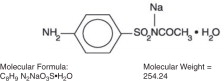
Sulfacetamide Sodium Ophthalmic Solution USP is a sterile, topical anti-bacterial agent for ophthalmic use. The active ingredient is represented by the following structural formula:
Chemical name: N-Sulfanilylacetamide monosodium salt monohydrate.
Ophthalmic Solution 30%, Each mL contains:
Active: Sulfacetamide Sodium 300 mg. Inactives: Sodium Thiosulfate 1.5 mg and Monobasic Sodium Phosphate as buffer. Preservatives: Methylparaben 0.5 mg and Propylparaben 0.1 mg.
Ophthalmic Solution 10%, Each mL contains:
Active: Sulfacetamide Sodium 100 mg. Inactives: Hydroxypropyl Methylcellulose 5 mg, Sodium Thiosulfate 3.1 mg and Monobasic Sodium Phosphate as buffer. Preservatives: Methylparaben 0.5 mg and Propylparaben 0.1 mg.
-
CLINICAL PHARMACOLOGY
Microbiology: The sulfonamides are bacteriostatic agents and the spectrum of activity is similar for all. Sulfonamides inhibit bacterial synthesis of dihydrofolic acid by preventing the condensation of the pteridine with aminobenzoic acid through competitive inhibition of the enzyme dihydropteroate synthetase. Resistant strains have altered dihydropteroate synthetase with reduced affinity for sulfonamides or produce increased quantities of aminobenzoic acid.
Topically applied sulfonamides are considered active against susceptible strains of the following common bacterial eye pathogens: Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus (viridans group), Haemophilus influenzae, Klebsiella species, and Enterobacter species.
Topically applied sulfonamides do not provide adequate coverage against Neisseria species, Serratia marcescens and Pseudomonas aeruginosa. A significant percentage of staphylococcal isolates are completely resistant to sulfa drugs.
-
INDICATIONS AND USAGE
For the treatment of conjunctivitis and other superficial ocular infections due to susceptible microorganisms and as an adjunctive in systemic sulfonamide therapy of trachoma:
Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus (viridans group), Haemophilus influenzae, Klebsiella species, and Enterobacter species.
Topically applied sulfonamides do not provide adequate coverage against Neisseria species, Serratia marcescens and Pseudomonas aeruginosa. A significant percentage of staphylococcal isolates are completely resistant to sulfa drugs.
- CONTRAINDICATIONS
-
WARNINGS
FOR TOPICAL EYE USE ONLY – NOT FOR INJECTION.
FATALITIES HAVE OCCURRED, ALTHOUGH RARELY, DUE TO SEVERE REACTIONS TO SULFONAMIDES INCLUDING STEVENS-JOHNSON SYN DROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS.
Sensitizations may recur when a sulfonamide is readministered, irrespective of the route of administration. Sensitivity reactions have been reported in individuals with no prior history of sulfonamide hypersensitivity. At the first sign of hypersensitivity, skin rash or other serious reaction, discontinue use of this preparation.
-
PRECAUTIONS
General: Prolonged use of topical anti-bacterial agents may give rise to overgrowth of nonsusceptible organisms including fungi. Bacterial resistance to sulfonamides may also develop.
Ophthlamic ointments may retard corneal wound healing.
The effectiveness of sulfonamides may be reduced by the para-aminobenzoic acid present in purulent exudates.
Sensitization may recur when a sulfonamide is readministered irrespective of the route of administration, and cross-sensitivity between different sulfonamides may occur.
At the first sign of hypersensitivity, increase in purulent discharge, or aggravation of inflammation or pain, the patient should discontinue use of the medication and consult a physician (see WARNINGS).
Information for patients: To avoid contamination, do not touch tip of container to eye, eyelid or any surface.
Carcinogenesis , Mutagenesis , Impairment of Fertility: No studies have been conducted in animals or in humans to evaluate the possibility of these effects with ocularly administered sulfacetamide. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides, and long-term oral administration of sulfonamides has resulted in thyroid malignancies in these animals.
Pregnancy: Pregnancy Category C. Animal reproduction studies have not been conducted with sulfonamide ophthalmic preparations. Kernicterus may occur in the newborn as a result of treatment of a pregnant woman at term with orally administered sulfonamides. There are no adequate and well controlled studies of sulfonamide ophthalmic preparations in pregnant women and it is not known whether topically applied sulfonamides can cause fetal harm when administered to a pregnant woman. This product should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: Systemically administered sulfonamides are capable of producing kernicterus in infants of lactating women. Because of the potential for the development of kernicterus in neonates, a decision should be made whether to discontinue nursing or discontinue the drug taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
Bacterial and fungal corneal ulcers have developed during treatment with sulfonamide ophthalmic preparations.
The most frequently reported reactions are local irritation, stinging and burning.
Less commonly reported reactions include non-specific conjunctivitis, conjunctival hyperemia, secondary infections and allergic reactions.
Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias (see WARNINGS).
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Sulfacetamide Sodium Ophthalmic Solution, USP 30%
NDC: 17478-242-12 15 mL dropper bottle, box of one.
Sulfacetamide Sodium Ophthalmic Solution, USP 10%
NDC: 17478-221-12 15 mL dropper bottle, box of one.
-
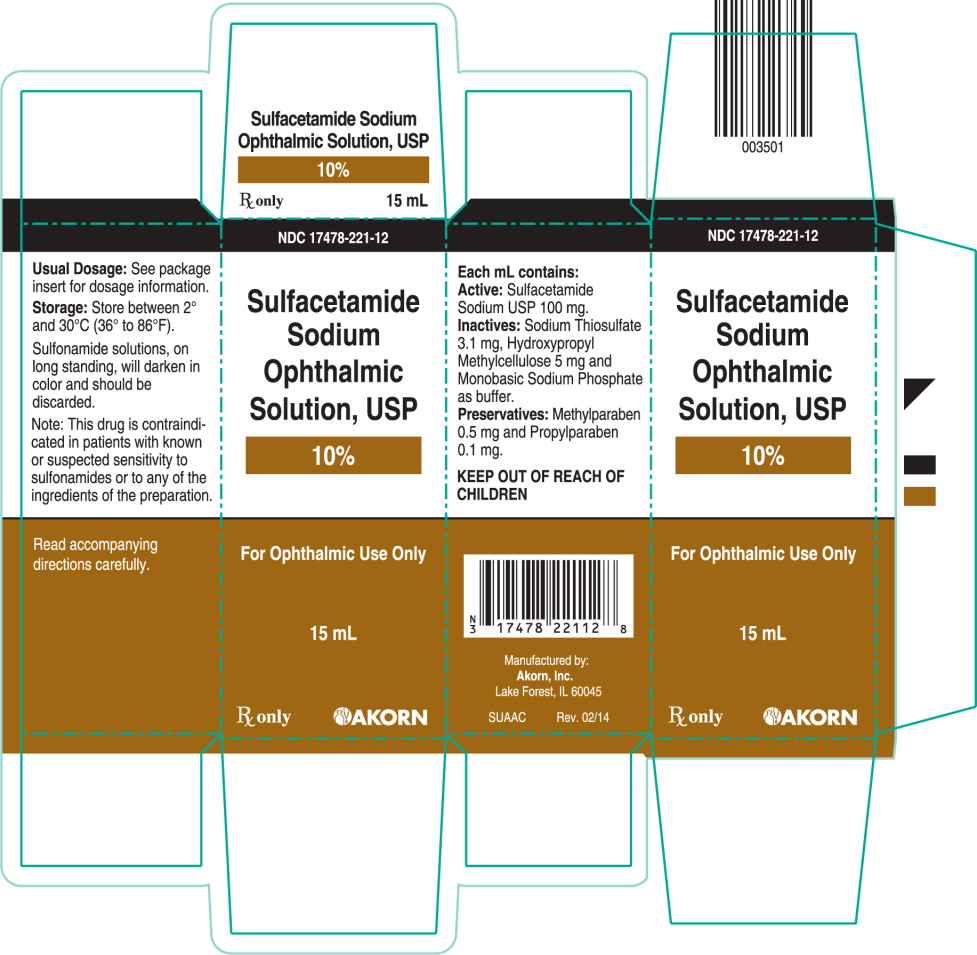
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 17478-221-12
Sulfacetamide
Sodium Ophthalmic
Solution, USP
10%
For Ophthalmic Use Only
Rx only 15 mL
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-221-12
Sulfacetamide
Sodium
Ophthalmic
Solution, USP
10%
For Ophthalmic Use Only
15 mL
Rx only Akorn logo
-
INGREDIENTS AND APPEARANCE
SULFACETAMIDE SODIUM
sulfacetamide sodium solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-221 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulfacetamide Sodium (UNII: 4NRT660KJQ) (Sulfacetamide - UNII:4965G3J0F5) Sulfacetamide Sodium 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Thiosulfate (UNII: HX1032V43M) Hypromelloses (UNII: 3NXW29V3WO) Sodium Phosphate, Monobasic (UNII: 3980JIH2SW) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-221-12 1 in 1 CARTON 09/11/2014 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040215 09/11/2014 SULFACETAMIDE SODIUM
sulfacetamide sodium solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-242 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulfacetamide Sodium (UNII: 4NRT660KJQ) (Sulfacetamide - UNII:4965G3J0F5) Sulfacetamide Sodium 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Thiosulfate (UNII: HX1032V43M) Sodium Phosphate, Monobasic (UNII: 3980JIH2SW) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-242-12 1 in 1 CARTON 09/11/2014 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040216 09/11/2014 Labeler - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 603980319 MANUFACTURE(17478-221, 17478-242) , ANALYSIS(17478-221, 17478-242) , STERILIZE(17478-221, 17478-242) , PACK(17478-221, 17478-242) , LABEL(17478-221, 17478-242)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.