XERMELO- telotristat ethyl tablet
Xermelo by
Drug Labeling and Warnings
Xermelo by is a Prescription medication manufactured, distributed, or labeled by TerSera Therapeutics LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XERMELO safely and effectively. See full prescribing information for XERMELO.
XERMELO® (telotristat ethyl) tablets, for oral use
Initial U.S. Approval: 2017RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Xermelo is a tryptophan hydroxylase inhibitor indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy. (1)
DOSAGE AND ADMINISTRATION
- The recommended dosage of Xermelo in adult patients is 250 mg three times daily for patients whose diarrhea is inadequately controlled by a SSA therapy. (2)
- Take Xermelo with food. (2)
- When short-acting octreotide is used in combination with Xermelo, administer short-acting octreotide at least 30 minutes after administering Xermelo. (2, 7.3)
- Discontinue Xermelo if severe constipation develops. (2, 5.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 250 mg telotristat ethyl (3)
CONTRAINDICATIONS
History of hypersensitivity to telotristat. (4)
WARNINGS AND PRECAUTIONS
Constipation: Xermelo reduces bowel movement frequency; monitor patients for constipation, and/or severe persistent or worsening abdominal pain. Discontinue Xermelo if constipation or abdominal pain develops. (5.1)
ADVERSE REACTIONS
Most common adverse reactions (≥5%) are nausea, headache, increased GGT, depression, flatulence, decreased appetite, peripheral edema, and pyrexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact TerSera Therapeutics LLC at 1-844-334-4035 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
CYP3A4 Substrates (e.g., midazolam) and CYP2B6 Substrates (e.g., bupropion, efavirenz): Efficacy of concomitant drugs may be decreased; monitor patients’ response and consider increasing the dosage of the concomitant drug, if necessary. (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Constipation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP3A4 Substrates
7.2 CYP2B6 Substrates
7.3 Short-Acting Octreotide
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dosage of Xermelo in adult patients is 250 mg three times daily for patients whose diarrhea is inadequately controlled by SSA therapy.
Administration
- Take Xermelo with food [see Clinical Pharmacology (12.3), Clinical Studies (14)].
- When short-acting octreotide is used in combination with Xermelo, administer short-acting octreotide at least 30 minutes after administering Xermelo [see Drug Interactions (7.3)].
- If a dose is missed, take the next dose at the regular time. Do not take 2 doses at the same time to make up for a missed dose.
- Discontinue Xermelo if severe constipation develops [see Warnings and Precautions (5.1)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Constipation
Xermelo reduces bowel movement frequency and may lead to constipation. Serious complications of constipation have been reported during clinical trials and postmarketing.
In a 12-week, placebo-controlled trial, in which patients had 4 or greater bowel movements per day, 2 out of 45 patients treated with a higher than recommended dosage of Xermelo reported constipation. In one patient the constipation was serious, resulting in hospitalization. During the 36-week extension period with higher than the recommended dosage of Xermelo, 10 of 115 patients reported constipation, with individual reports of intestinal perforation, obstruction, and fecaloma. In another 12-week, placebo-controlled trial in which patients had less than 4 bowel movements per day, 4 out of 25 patients treated with the recommended dosage of Xermelo reported constipation.
Serious complications of constipation in patients treated with Xermelo at the recommended dosage (e.g., intestinal obstruction) have also been reported in the postmarket setting. Most patients had additional risk factors, including underlying disease and concomitant constipating medications.
Given that patients with metastatic carcinoid tumors may have impaired integrity of the gastrointestinal tract wall, monitor for the development of constipation and/or severe, persistent, or worsening abdominal pain in patients taking Xermelo. Discontinue Xermelo if severe constipation or severe persistent or worsening abdominal pain develops [see Dosage and Administration (2), Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Xermelo was studied in a double-blind, placebo-controlled clinical trial of 90 patients with metastatic neuroendocrine tumors and carcinoid syndrome diarrhea. Patients reported between 4 to 12 bowel movements daily despite the use of SSA therapy at a stable dose for at least 3 months [see Clinical Studies (14)]. Placebo or Xermelo 250 mg was administered three times daily for 12 weeks. Concomitant anti-diarrheal medications (e.g., loperamide) were used by 43% (36% and 51% in the placebo and Xermelo group, respectively), pancreatic enzyme replacement medications by 39% (36% and 42% in the placebo and Xermelo group, respectively), and opioid analgesics by 29% (24% and 33% in the placebo and Xermelo group, respectively) of patients during the 12-week double-blind period of the trial.
Table 1 below lists adverse reactions occurring at an incidence of at least 5% in the Xermelo group (N=45) and at an incidence greater than placebo (N=45) during the 12-week placebo-controlled period of the trial.
Table 1: Percent Common Adverse Reactionsa by Treatment Group at 12-Weeks in a Double-Blind Placebo-Controlled Clinical Trial of Patients with Carcinoid Syndrome Diarrhea a incidence of at least 5% in the Xermelo group and at an incidence greater than placebo
b including depression, depressed mood and decreased interest
Adverse Reaction Xermelo
250 mg Three Times Daily, N=45
(%)Placebo,
N=45
(%)Nausea 13 11 Headache 11 4 Increased gamma-glutamyl-transferase (GGT) 9 0 Depressionb 9 7 Peripheral edema 7 2 Flatulence 7 2 Decreased appetite 7 4 Pyrexia 7 4 In another placebo-controlled clinical trial of patients with carcinoid syndrome diarrhea and less than 4 bowel movements per day, the following additional adverse reactions, not listed in Table 1, of abdominal pain (including upper and lower abdominal pain, abdominal distention and gastrointestinal pain) and constipation were reported in at least 5% of patients in the Xermelo treated group and at an incidence greater than placebo [see Warnings and Precautions (5.1)].
Less Common Adverse Reactions:
The following is a list of adverse reactions occurring in less than 5% of patients receiving Xermelo during the 12-week placebo-controlled period of the clinical trial:
Investigations: increased alkaline phosphatase, increased alanine aminotransferase, and increased aspartate aminotransferase.
Fecaloma was reported in one patient treated with Xermelo (at a higher than recommended dosage) during the 36-week open-label extension period following the 12-week double-blind period of the trial.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Xermelo. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal: intestinal obstruction [see Warnings and Precautions (5.1)]
Immune system disorders: angioedema
Skin and subcutaneous tissue disorders: pruritis, rash
-
7 DRUG INTERACTIONS
7.1 CYP3A4 Substrates
Concomitant use of Xermelo may decrease the efficacy of drugs that are CYP3A4 substrates (e.g., midazolam) by decreasing their systemic exposure. Monitor patients’ response to CYP3A4 substrates when co-administered with Xermelo and consider increasing the dosage of the interacting drug, if necessary [see Clinical Pharmacology (12.3)].
7.2 CYP2B6 Substrates
Concomitant use of Xermelo may decrease the efficacy of drugs that are CYP2B6 substrates (e.g., bupropion, efavirenz) by decreasing their systemic exposure. Monitor patients’ response to CYP2B6 substrates when co-administered with Xermelo and consider increasing the dosage of the interacting drug, if necessary [see Clinical Pharmacology (12.3)].
7.3 Short-Acting Octreotide
Concurrent administration of short-acting octreotide with Xermelo significantly decreased the systemic exposure of telotristat ethyl and telotristat, the active metabolite. If treatment with short-acting octreotide is needed in combination with Xermelo, administer short-acting octreotide at least 30 minutes after administration of Xermelo [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no human data with Xermelo use in pregnant women to inform a drug-associated risk. In animal reproduction studies, no effects on embryo-fetal development were observed with the administration of oral telotristat ethyl to rats during organogenesis at doses up to 750 mg/kg/day (approximately 9 times the exposure at the RHD [recommended human dose]). Treatment of pregnant rabbits with oral telotristat ethyl during organogenesis produced maternal toxicity and post-implantation loss at doses of 250 mg/kg/day or higher (approximately 15 times the exposure at the RHD), and reduced fetal weight at 500 mg/kg/day (approximately 33 times the exposure at the RHD). In a pre-/postnatal development study, an increased incidence of mortality in rat offspring was observed during postnatal days 0 to 4 at the maternal oral dose of 500 mg/kg/day (approximately 5 times the exposure at the RHD), given during organogenesis through lactation (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal data
An embryo-fetal development study performed in rats with oral telotristat ethyl at doses up to 750 mg/kg/day (approximately 9 times the AUC [area under the plasma concentration-time curve] for the active metabolite at the RHD) during organogenesis produced no harm to embryo-fetal development.
In pregnant rabbits treated orally with telotristat ethyl during organogenesis, an increased incidence of post-implantation loss at doses of 250 and 500 mg/kg/day (approximately 15 times the AUC for the active metabolite at RHD) and a decrease in fetal weight at 500 mg/kg/day (approximately 33 times the AUC for the active metabolite at the RHD) was observed. The adverse effects on embryo-fetal development were associated with maternal toxicity (impaired weight gain and/or mortality) at 250 and 500 mg/kg/day. No adverse effects on embryo-fetal development were observed at 125 mg/kg/day (approximately 5 times the AUC for the active metabolite at the RHD).
A pre-/postnatal development study was conducted in rats using oral administration of 100, 200, and 500 mg/kg/day telotristat ethyl during organogenesis through lactation. An increased incidence of pup mortality was observed during postnatal days 0 to 4 at the maternal dose of 500 mg/kg/day (approximately 5 times the AUC for the active metabolite at the RHD). No developmental abnormalities or effects on growth, learning and memory, or reproductive performance were observed through maturation of offspring at maternal doses of up to 500 mg/kg/day in surviving offspring.
8.2 Lactation
Risk Summary
There are no data on the presence of telotristat ethyl in human or animal milk, the effects on the breastfed infant, or the effects on milk production. The effects of local gastrointestinal and systemic exposure to telotristat ethyl on breastfed infants are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Xermelo and any potential adverse effects on the breastfed infant from Xermelo or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Xermelo in pediatric patients have not been established.
8.5 Geriatric Use
Of 45 patients in a clinical trial of Xermelo, 19 (42%) patients were 65 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dosage adjustment of Xermelo is necessary in patients with mild, moderate or severe renal impairment who are not requiring dialysis.
There is no information on Xermelo in patients with end-stage renal disease who require dialysis (eGFR <15 mL/min/1.73 m2).
8.7 Hepatic Impairment
Systemic exposure of telotristat ethyl and its active metabolite, telotristat, were substantially increased in patients with moderate hepatic impairment (Child-Pugh Class B) and severe hepatic impairment (Child-Pugh C) (3.2- and 5.0-fold, respectively) compared to patients with normal hepatic function [see Clinical Pharmacology (12.3)]. Xermelo is not recommended in patients with moderate and severe hepatic impairment.
No dosage adjustment of Xermelo is necessary in patients with mild hepatic impairment (Child-Pugh A); however, additional monitoring of Xermelo-associated adverse reactions (e.g., constipation) is recommended in these patients [see Warnings and Precautions (5.1)].
-
11 DESCRIPTION
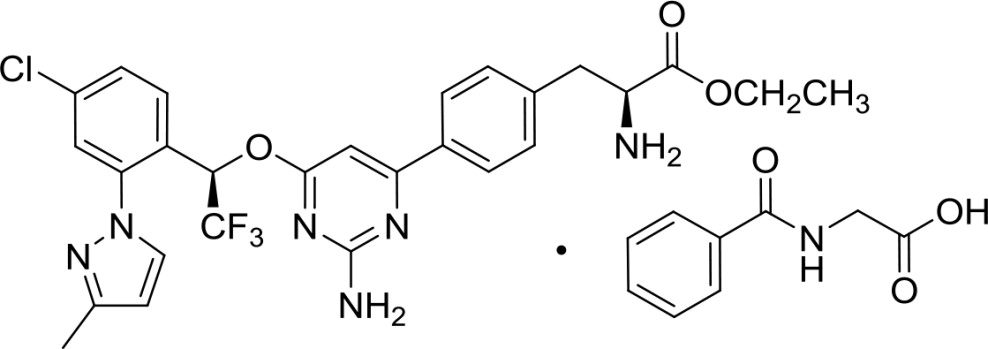
Xermelo (telotristat ethyl) tablets contain telotristat ethyl as telotristat etiprate, a tryptophan hydroxylase inhibitor. Telotristat etiprate is the hippurate salt of telotristat ethyl [(S)-ethyl 2-amino-3-(4-(2-amino-6-((R)-1-(4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)pyrimidin-4-yl)phenyl)propanoate], which undergoes hydrolysis to the active metabolite, (S)-2-amino-3-(4-(2-amino-6-((R)-1-(4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)pyrimidin-4-yl)phenyl)propanoic acid.
The molecular formula of telotristat etiprate is C27H26ClF3N6O3 C9H9NO3 and its molecular weight is 754.2. The molecular weight of the free base (telotristat ethyl) is 575.0.
Chemical Structure:
Telotristat etiprate is a white to off-white solid. The solubility is a function of pH at 25°C; at pH 1 (0.1N HCl), the solubility is greater than 71 mg/mL, at pH 3 phosphate buffer, the solubility is 0.30 mg/mL, at a pH of 5 to 9, the solubility is negligible. In organic solvents, telotristat etiprate is freely soluble in methanol, soluble in acetone, and sparingly soluble in ethanol.
Each Xermelo tablet contains 250 mg of telotristat ethyl (free base) which is equivalent to 328 mg telotristat etiprate. The inactive ingredients of Xermelo tablets include: colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, lactose anhydrous, macrogol/PEG, magnesium stearate, polyvinyl alcohol [part hydrolyzed], talc and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Telotristat, the active metabolite of telotristat ethyl, is an inhibitor of tryptophan hydroxylase, which mediates the rate limiting step in serotonin biosynthesis. The in vitro inhibitory potency of telotristat towards tryptophan hydroxylase is 29 times higher than that of telotristat ethyl. Serotonin plays a role in mediating secretion, motility, inflammation, and sensation of the gastrointestinal tract, and is over-produced in patients with carcinoid syndrome. Through inhibition of tryptophan hydroxylase, telotristat and telotristat ethyl reduce the production of peripheral serotonin, and the frequency of carcinoid syndrome diarrhea.
12.2 Pharmacodynamics
In healthy subjects, telotristat ethyl 500 mg three times daily (twice the recommended dosage) for 14 days decreased whole blood serotonin and 24-hour urinary 5-hydroxyindolacetic acid (u5-HIAA) from baseline. A decrease in 24-hour u5-HIAA was observed as early as after 5 days of treatment.
In patients with metastatic neuroendocrine tumors and carcinoid syndrome diarrhea, 24-hour u5-HIAA decreased from baseline following 6 and 12 weeks of treatment with Xermelo 250 mg three times a day, whereas placebo did not decrease u5-HIAA.
12.3 Pharmacokinetics
Absorption
After a single oral dose of telotristat ethyl to healthy subjects, telotristat ethyl was absorbed and metabolized to its active metabolite, telotristat. Peak plasma concentrations of telotristat ethyl were achieved within 0.5 to 2 hours, and those of telotristat within 1 to 3 hours. Plasma concentrations thereafter declined in a biphasic manner. Following administration of a single 500 mg dose of telotristat ethyl (twice the recommended dosage) under fasted conditions in healthy subjects, the mean Cmax and AUC0-inf were 4.4 ng/mL and 6.23 nghr/mL, respectively for telotristat ethyl. The mean Cmax and AUC0-inf were 610 ng/mL and 2320 nghr/mL, respectively for telotristat. Peak plasma concentrations and AUC of telotristat ethyl and telotristat appeared to be dose proportional following administration of a single dose of telotristat ethyl in the range of 100 mg (0.4 times the lowest recommended dose to 1000 mg [4 times the highest recommended dose]) under fasted conditions.
Following multiple-dose administration of telotristat ethyl 500 mg three times daily, there was negligible accumulation at steady state for both telotristat ethyl and telotristat.
In patients with metastatic neuroendocrine tumors and carcinoid syndrome diarrhea treated with SSA therapy, the median Tmax for telotristat ethyl and telotristat was approximately 1 and 2 hours, respectively. Following administration of 500 mg telotristat ethyl three times daily, with meals in patients, the mean Cmax and AUC0-6hr were approximately 7 ng/mL and 22 nghr/mL, respectively, for telotristat ethyl. The mean Cmax and AUC0-6hr were approximately 900 ng/mL and 3000 nghr/mL, respectively for telotristat. The pharmacokinetic parameters for both telotristat ethyl and telotristat were highly variable with about 55% coefficient of variation.
Food Effect
Administration of a single 500 mg dose of Xermelo (twice the recommended dose) with food resulted in higher exposure to both telotristat ethyl and telotristat. The systemic exposure to telotristat ethyl, was significantly increased following administration with a high-fat meal, with Cmax, and AUC0-inf being 112%, and 264% higher, respectively compared to the fasted state. Following administration of a single 500 mg dose of telotristat ethyl under fed conditions in healthy subjects, the mean Cmax and AUC0-inf were 10.5 ng/mL and 21.6 nghr/mL, respectively for telotristat ethyl. The Cmax and AUC0-inf values for telotristat were also increased by 47% and 33%, respectively, with a high-fat meal compared to the fasted state. The mean Cmax and AUC0-inf were 908 ng/mL and 2980 nghr/mL, respectively for telotristat under fed conditions [see Dosage and Administration ( 2)].
Distribution
Both telotristat ethyl and telotristat are greater than 99% bound to human plasma proteins.
In vitro data suggests that telotristat is a substrate of P-glycoprotein.
Elimination
Following a single 500 mg oral dose of telotristat ethyl in healthy subjects, the apparent half-life was approximately 0.6 hours for telotristat ethyl and 5 hours for telotristat. The apparent total clearance at steady state (CL/Fss) following oral dosing with telotristat ethyl 500 mg three times daily for 14 days (twice the recommended dosage) in healthy subjects was 2.7 and 152 L/hr for telotristat ethyl and telotristat, respectively.
Metabolism
After oral administration, telotristat ethyl undergoes hydrolysis via carboxylesterases to telotristat, its active metabolite. Telotristat is further metabolized. Among the metabolites of telotristat, the systemic exposure to an acid metabolite of oxidative deaminated decarboxylated telotristat was about 35% of that of telotristat. In vitro data suggest that telotristat ethyl and telotristat are not substrates for CYP enzymes.
Specific Populations
Age and Sex
Population pharmacokinetic analysis indicated that age (18 to 83 years) and sex do not affect the pharmacokinetics of telotristat.
Renal Impairment
Exposure to telotristat ethyl and its active metabolite, telotristat, was similar in patients with severe renal impairment or end-stage renal disease without dialysis (eGFR < 30 mL/min/1.73 m2) compared with subjects with normal renal function following a single oral dose of Xermelo [see Use in Specific Populations (8.6)]. Xermelo was not studied in patients with end-stage renal disease who require dialysis (eGFR < 15 mL/min/1.73 m2).
Hepatic Impairment
Following a single dose of Xermelo 500 mg, systemic exposure (AUC0-last) to telotristat ethyl was 2.3- and 3.2-fold higher in subjects with mild (Child-Pugh A) and moderate (Child-Pugh B) hepatic impairment, respectively, than in subjects with normal hepatic function. In subjects with severe (Child-Pugh C) hepatic impairment, following a single Xermelo 250 mg dose, systemic exposure (AUC0-last), was 4-fold higher than in subjects with normal hepatic function. In these same studies, AUC0-last for telotristat (active metabolite) was 2.4-, 3.5-, and 5-fold higher in subjects with mild, moderate, and severe hepatic impairment, respectively [see Use in Specific Populations (8.7)].
In patients with metastatic neuroendocrine tumors and carcinoid syndrome diarrhea, population pharmacokinetic analysis indicated that mild hepatic impairment (defined as total bilirubin greater than 1 to 1.5 times the upper limit of normal [ULN] or AST greater than the ULN) did not affect the pharmacokinetics of telotristat.
Drug Interaction Studies
Effect of Telotristat Ethyl on Other Drugs
In vitro studies
Based on in vitro studies, the potential for telotristat ethyl, telotristat, and the acid metabolite of telotristat to inhibit major CYP enzymes (1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4/5) and to induce CYP1A2 is low at the recommended dosage of Xermelo.
Based on in vitro studies, potential induction of CYP2B6 in vivo by Xermelo cannot be ruled out [see Drug Interactions (7.2)].
In vitro telotristat ethyl, but not telotristat, inhibited breast cancer resistance protein (BCRP) at the clinically relevant concentrations. However, in vivo drug interaction potential via inhibition of BCRP is low based on in vitro studies and in vivo findings.
Based on in vitro studies, in vivo drug interaction potential via inhibition of organic cation transporter 1 (OCT1), OCT2, organic anion transporter 1 (OAT1), OAT3, organic anion transporting polypeptide 1B1 (OATP1B1), OATP1B3, or bile salt export pump (BSEP) transporters by telotristat ethyl and telotristat is low at the recommended dosage.
Based on in vitro study results, the potential for the acid metabolite of telotristat to inhibit P-gp, BCRP, OCT1, OCT2, OAT1, OAT3, OATP1B1, OATP1B3, BSEP, and MRP2 transporters is low at the recommended dosage.
Midazolam (sensitive CYP3A4 substrate)
Following administration of multiple doses of telotristat ethyl, the systemic exposure to concomitant midazolam was significantly decreased. When 3 mg midazolam was co-administered orally after 5 day treatment with telotristat ethyl 500 mg three times daily (twice the recommended dosage), the mean Cmax, and AUC0-inf for midazolam were decreased by 25%, and 48%, respectively, compared to administration of midazolam alone. The mean Cmax, and AUC0-inf for the active metabolite, 1'-hydroxymidazolam, were also decreased by 34%, and 48%, respectively. The reduction in the systemic exposure to both midazolam and its active metabolite suggests that the glucuronidation of 1'-hydroxymidazolam may have been increased by telotristat ethyl [see Drug Interactions (7.1)].
Fexofenadine (sensitive P-gp substrate)
In vitro telotristat ethyl, but not telotristat, inhibited P-glycoprotein (P-gp). In healthy volunteers, the Cmax and AUC of fexofenadine increased by 16% when a single 180 mg dose of fexofenadine was co-administered orally with the final dose of telotristat ethyl 500 mg administered three times daily (twice the recommended dosage) for 5 days. Clinically meaningful interactions with P-gp substrates are unlikely.
Effect of Other Drugs on Telotristat Ethyl
Short-Acting Octreotide
The mean Cmax and AUC0-last of telotristat ethyl were decreased by 86% and 81%, respectively, following administration of a single 500 mg dose of Xermelo (twice the recommended dose), co-administered with short-acting octreotide 200 mcg injected subcutaneously in healthy subjects. The mean Cmax and AUC0-last of telotristat were decreased by 79% and 68%, respectively.
Gastric Acid Reducers (Proton Pump Inhibitor and H2-Receptor Antagonist)
Omeprazole: The Cmax and AUCinf of telotristat ethyl were increased by 68% and 185%, respectively, when a single 250 mg dose of Xermelo was coadministered with a 40 mg dose of omeprazole once daily compared to administration of Xermelo alone. No significant change (<9%) in AUC and Cmax of the active metabolite, telotristat, was observed following coadministration of Xermelo with omeprazole compared to administration of Xermelo alone. These changes in exposure of telotristat ethyl and telotristat are not considered to be clinically meaningful.
Famotidine: The Cmax and AUCinf of telotristat ethyl were increased by 22% and 111%, respectively, when a single 250 mg dose of Xermelo was coadministered with a 40 mg dose of famotidine twice daily. No significant change (<5%) in AUC and Cmax to the active metabolite, telotristat, was observed following coadministration of Xermelo with famotidine compared to administration of Xermelo alone. These changes in exposure are not considered to be clinically meaningful.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 26-week study in transgenic (Tg.rasH2) mice, telotristat ethyl was not tumorigenic at oral doses up to 300 mg/kg/day (approximately 12 to 19 times the AUC for the active metabolite at the RHD).
In a 2-year carcinogenicity study in Sprague-Dawley rats, telotristat ethyl was not tumorigenic at oral doses up to 170 mg/kg/day (approximately 2 to 5 times the AUC for the active metabolite at the RHD).
Telotristat ethyl was negative in the in vitro Ames test, the in vitro chromosomal aberration test using Chinese hamster ovary cells, and the in vivo rat micronucleus test.
Telotristat ethyl at oral doses up to 500 mg/kg/day (approximately 5 times the AUC for the active metabolite at the RHD) was found to have no effect on fertility and reproductive performance of male or female rats.
-
14 CLINICAL STUDIES
A 12-week double-blind, placebo-controlled, randomized, multicenter trial of Xermelo was conducted in adult patients with a well-differentiated metastatic neuroendocrine tumor and carcinoid syndrome diarrhea who were having between 4 to 12 daily bowel movements despite the use of SSA therapy at a stable dose for at least 3 months. Patients were randomized to placebo or treatment with Xermelo 250 mg three times daily.
Study medication was administered within 15 minutes before or within 1 hour after a meal or snack [see Dosage and Administration (2)]. All patients were required to stay on their baseline SSA regimen and were allowed to use rescue medication (short-acting octreotide) and antidiarrheals (e.g., loperamide) for symptomatic relief. A total of 90 patients were evaluated for efficacy. The mean age of the population was 63 years of age (range 37 to 83 years), 50% were male, and 90% were White.
The primary efficacy endpoint was the change from baseline in the number of daily bowel movements averaged over the 12-week treatment period. The analysis results can be found in Table 2 below. The average was based on the number of days with valid, non-missing data. When a patient had more than 6 weeks of missing data, the change from baseline was considered equal to zero. A week of missing data was defined as a patient missing at least 4 days of diary data in that week.
Table 2: Change from Baseline in Bowel Movements/Day Averaged Over 12 Weeks in Adult Patients with Carcinoid Syndrome Diarrhea Parameter Xermelo 250 mg three times daily Placebo CL=confidence limit; SD=standard deviation.
a Baseline Bowel Movements/Day was assessed over the 3-4 week screening/run-in period.
b Statistical tests used a blocked 2-sample Wilcoxon Rank Sum statistic (van Elteren test) stratified by the u5-HIAA stratification at randomization. CLs were based on the Hodges-Lehmann estimator of the median paired difference.
c p<0.001
Bowel Movements/Day At Baselinea Number of Patients 45 45 Baseline Mean (SD)
Median (Min, Max)6.1 (2.1)
5.5 (3.5, 13.0)5.2 (1.4)
5.1 (3.5, 9.0)Change From Baseline In Bowel Movements/Day Averaged Over 12 Weeks Change Averaged over 12 Weeks: Mean (SD)
Median (Min, Max)˗1.4 (1.4)
-1.3 (-6.1, 1.6)˗0.6 (0.8)
-0.6 (-2.7, 0.8)Estimate of Treatment Difference (97.5% CL)b ˗0.8c
(˗1.3, ˗0.3)--- In the 12-week study, a difference in average weekly reductions in bowel movement frequency between Xermelo and placebo was observed as early as 1 to 3 weeks, and persisted for the remaining 9 weeks of the study.
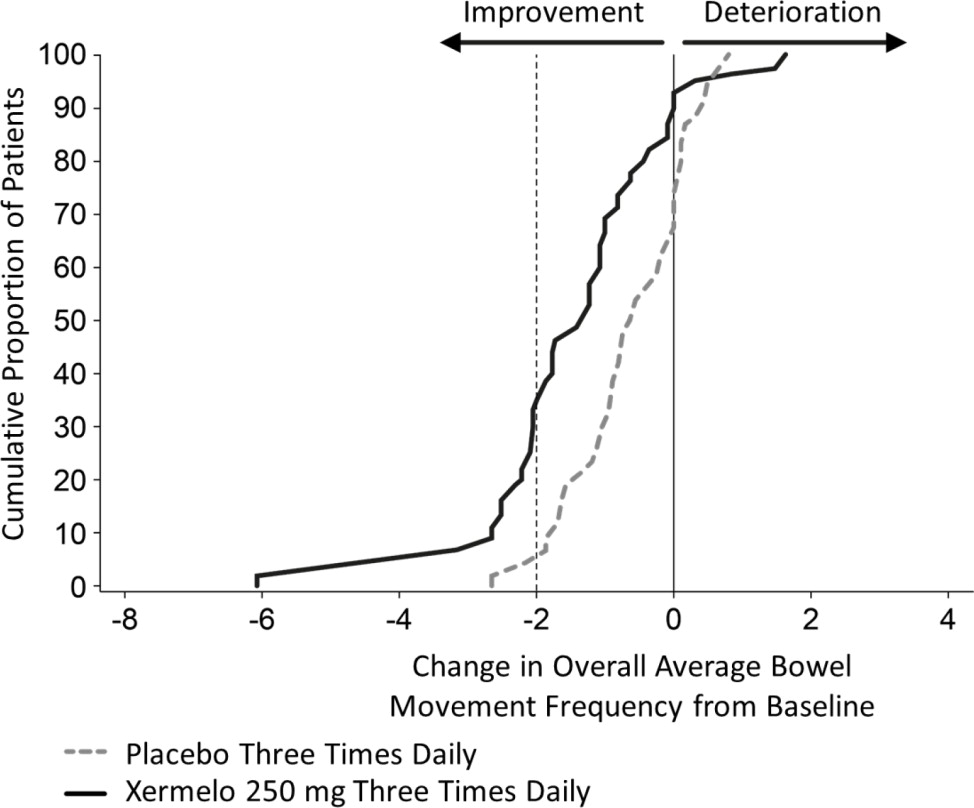
To aid in the interpretation of the bowel movement reduction results, the proportion of patients reporting any particular level of reduction in overall average bowel movement frequency is depicted in Figure 1 below. For example, 33% of patients randomized to Xermelo and 4% of patients randomized to placebo experienced a reduction in overall average bowel movements from baseline of at least 2 bowel movements per day.
Figure 1: Cumulative Proportion of Patients with Carcinoid Syndrome Diarrhea Reporting Change in Overall Average Bowel Movement Frequency
Other symptoms of carcinoid syndrome (abdominal pain or flushing) did not show improvement in the comparison of Xermelo to placebo.
The average number of daily short-acting octreotide injections used for rescue therapy over the 12-week double-blind treatment period was 0.3 and 0.7 in the Xermelo and placebo groups, respectively. In the subgroup of patients who received short-acting octreotide injections, observed reductions in the number of bowel movements per day and treatment differences were generally consistent with the reductions and differences observed in patients who did not receive rescue therapy, and were similar to the overall data presented in Table 2 above [see Dosage and Administration (2), Drug Interactions (7.2)].
A third randomized treatment arm of Xermelo 500 mg three times daily did not demonstrate additional treatment benefit on the primary endpoint and had a greater incidence of adverse reactions than Xermelo 250 mg three times daily. Therefore, Xermelo 500 mg three times daily is not recommended [see Dosage and Administration (2)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
250 mg tablet: white to off-white coated oval tablet with “T-E” debossed on one side and “250” debossed on the other side.
Xermelo is dispensed in a monthly case for a total of 28 days of therapy. Each monthly case contains four weekly packs. Each weekly pack contains 21 tablets.
- NDC: 70720-125-85: Monthly case of 84 tablets. Each child resistant weekly pack contains twenty-one 250 mg tablets.
-
17 PATIENT COUNSELING INFORMATION
Advise patients:
- If they experience severe constipation or severe persistent or worsening abdominal pain, to discontinue Xermelo and contact their healthcare provider [see Warnings and Precautions (5.1)].
- To take Xermelo with food [see Clinical Pharmacology (12.3), Clinical Studies (14)].
- When short-acting octreotide is used in combination with Xermelo, administer short-acting octreotide at least 30 minutes after administering Xermelo [see Drug Interactions (7.3)].
- If a dose is missed, take the next dose at the regular time. Do not take 2 doses at the same time to make up for a missed dose.
Distributed by:
TerSera Therapeutics LLC, Deerfield, IL 60015©2022 TerSera Therapeutics LLC
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 3x7 weekly pack
TO OPEN:
1 Press & hold button
2 Pull out card
WEEKLY PACK
21 TABLETS
Rx OnlyXERMELO®
(telotristat ethyl) tablets
250 mg
per tabletTerSera
therapeuticsFor oral use only.
As with all medications, keep
out of reach of children.NDC: 70720-125-22
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 28 Day Case Label
NDC: 70720-125-85 28 DAY CASE
Rx Only 84 Tablets
XERMELO®
(telotristat ethyl) tablets250 mg
per tabletTerSera
therapeuticsFor oral use only.
As with all medications, keep out of reach of children.
-
INGREDIENTS AND APPEARANCE
XERMELO
telotristat ethyl tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70720-125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength telotristat ethyl (UNII: 8G388563M7) (telotristat - UNII:381V4FCV2Z) telotristat ethyl 250 mg Inactive Ingredients Ingredient Name Strength anhydrous lactose (UNII: 3SY5LH9PMK) hydroxypropyl cellulose (90000 wamw) (UNII: UKE75GEA7F) croscarmellose sodium (UNII: M28OL1HH48) magnesium stearate (UNII: 70097M6I30) silicon dioxide (UNII: ETJ7Z6XBU4) polyvinyl alcohol, unspecified (UNII: 532B59J990) titanium dioxide (UNII: 15FIX9V2JP) polyethylene glycol, unspecified (UNII: 3WJQ0SDW1A) talc (UNII: 7SEV7J4R1U) Product Characteristics Color white (white to off-white) Score no score Shape OVAL (OVAL) Size 17mm Flavor Imprint Code T;E;250 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70720-125-85 4 in 1 CASE 10/01/2021 1 NDC: 70720-125-22 1 in 1 BOX 1 21 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208794 10/05/2020 Labeler - TerSera Therapeutics LLC (080226115)
Trademark Results [Xermelo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XERMELO 87320213 not registered Live/Pending |
Lexicon Pharmaceuticals, Inc. 2017-01-31 |
 XERMELO 86013323 5200148 Live/Registered |
Lexicon Pharmaceuticals, Inc. 2013-07-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.