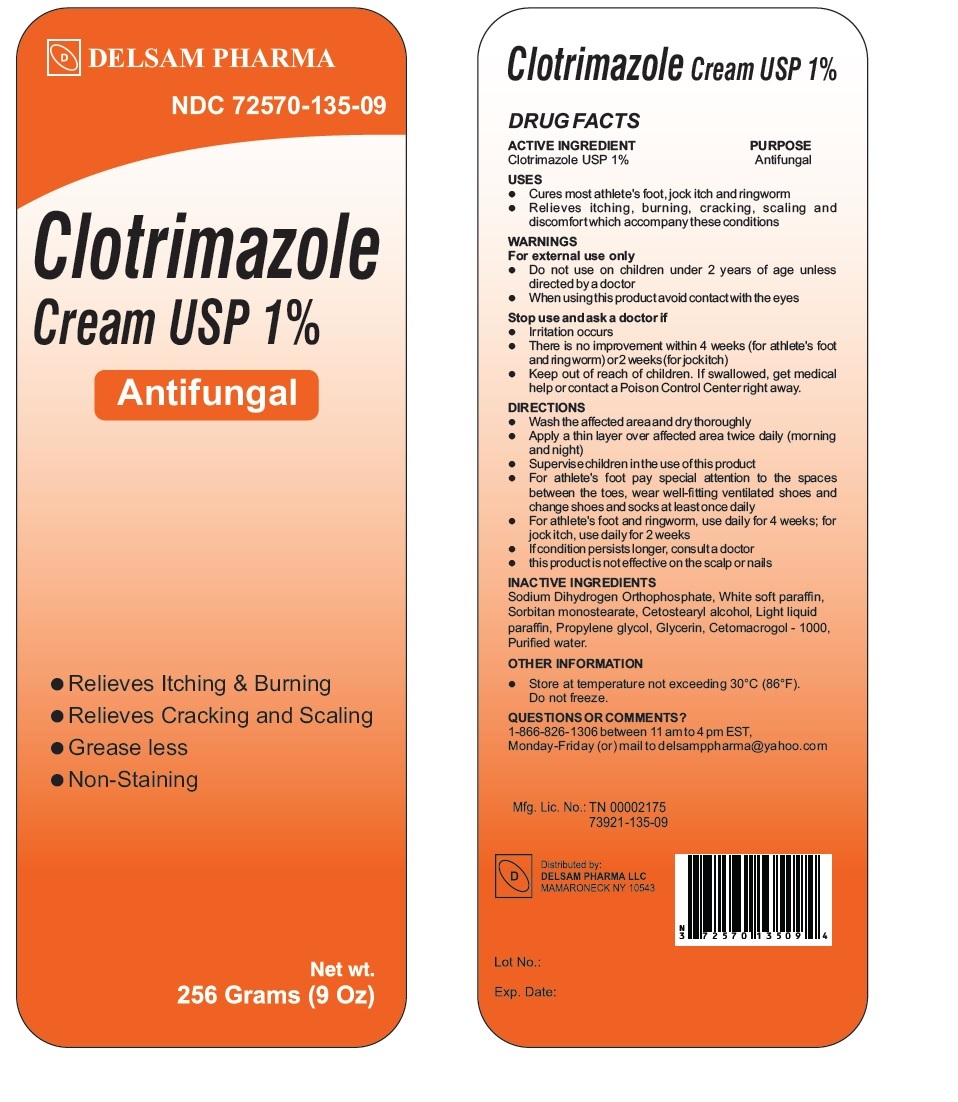
Clotrimazole Cream USP 1%
CLOTRIMAZOLE 1% by
Drug Labeling and Warnings
CLOTRIMAZOLE 1% by is a Otc medication manufactured, distributed, or labeled by Delsam Pharma Llc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CLOTRIMAZOLE 1%- clotrimazole cream
Delsam Pharma Llc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Clotrimazole Cream USP 1%
USES
Cures most athlete's foot, jock itch and ringworm
Relieves itching, burning, cracking, scaling and discomfort which accompany these conditions
WARNINGS
For external use only
Do not use on children under 2 years of age unless directed by a doctor
When using this product avoid contact with the eyes
Stop use and ask a doctor if
Irritation occurs
There is no improvement within 4 weeks (for athlete's foot and ring worm) or 2 weeks (for jock itch)
DIRECTIONS
Wash the affected area and dry thoroughly
Apply a thin layer over affected area twice daily (morning and night)
Supervise children in the use of this product
For athlete's foot pay special attention to the spaces between the toes, wear well-fitting ventilated shoes and change shoes and socks at least once daily
For athlete's foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
If condition persists longer, consult a doctor
this product is not effective on the scalp or nails
INACTIVE INGREDIENTS
Sodium Dihydrogen Orthophosphate, White soft paraffin, Sorbitan monostearate, Cetostearyl alcohol, Light liquid paraffin, Propylene glycol, Glycerin, Cetomacrogol -1000, Purified water.
QUESTIONS OR COMMENTS?
1-866-826-1306 between 11 am to 4 pm EST, Monday-Friday (or) mail to delsamppharma@yahoo.com
| CLOTRIMAZOLE 1%
clotrimazole cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Delsam Pharma Llc (081369679) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.