RYONCIL- remestemcel-l-rknd kit
RYONCIL by
Drug Labeling and Warnings
RYONCIL by is a Prescription medication manufactured, distributed, or labeled by Mesoblast, Mesoblast, Inc., LONZA BIOLOGICS TUAS PTE. LTD., Integrated Commercialization Solutions, LLC, WuXi Advanced Therapies Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RYONCIL® safely and effectively. See full prescribing information for RYONCIL®.
RYONCIL® (remestemcel-L-rknd) suspension for intravenous infusion
Initial U.S. Approval: December 2024RECENT MAJOR CHANGES
How Supplied/Storage and Handling (16) 09/2025 INDICATIONS AND USAGE
RYONCIL is an allogeneic bone marrow-derived mesenchymal stromal cell (MSC) therapy indicated for the treatment of steroid-refractory acute graft versus host disease (SR-aGvHD) in pediatric patients 2 months of age and older. (1)
DOSAGE AND ADMINISTRATION
For intravenous use only. (2)
- The recommended dosage of RYONCIL is 2 × 106 MSC/kg body weight per intravenous infusion given twice per week for 4 consecutive weeks. Infusions should be administered at least 3 days apart.
- Assess response 28 ± 2 days after the first dose and administer further treatment as appropriate as described in the table below.
Recommended Treatment Based on Day 28 Response Response Recommendation Complete Response (CR) No further treatment with RYONCIL Partial or Mixed Response Repeat administration of RYONCIL once a week for additional 4 weeks (4 infusions total) No Response Consider alternative treatments Recurrence of GvHD after CR Repeat administration of RYONCIL twice a week for an additional 4 consecutive weeks (8 infusions total) DOSAGE FORMS AND STRENGTHS
RYONCIL is available as a cell suspension for intravenous infusion in a target concentration of 6.68 X 106 MSCs per mL in 3.8 mL contained in a 6 mL cryovial. (3)
CONTRAINDICATIONS
Known hypersensitivity to dimethyl sulfoxide (DMSO) or Porcine and Bovine proteins. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity/Acute Infusion reactions: Monitor for hypersensitivity reactions during infusion and premedicate with corticosteroids and antihistamines. (5.1)
Transmission of Infectious Agents: RYONCIL may transmit infectious agents. (5.2)
Ectopic Tissue Formation: Ectopic tissue formation may occur following treatment with RYONCIL. (5.3)
ADVERSE REACTIONS
The most common non-laboratory adverse reactions (incidence ≥20%) are: viral infectious disorders, bacterial infectious disorders, infection – pathogen unspecified, pyrexia, hemorrhage, edema, abdominal pain and hypertension (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Mesoblast at toll-free phone #1-844-889-MESO (6376) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity and Acute Infusion Reactions
5.2 Transmission of Infectious Agents
5.3 Ectopic Tissue Formation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intravenous use only.
2.1 Recommended Dosage
- The recommended dosage of RYONCIL is 2 × 106 mesenchymal stromal cells (MSC)/kg body weight per intravenous infusion given twice a week for 4 consecutive weeks for a total of 8 infusions. Administer infusions at least 3 days apart.
- Assess response 28 ± 2 days after the first dose and administer further treatment as appropriate as described in Table 1 based on Day 28 response.
Table 1: Recommended Treatment based on Day 28 Response Response Recommendation - * Partial response defined as organ improvement of at least one stage without worsening in any other organ, whereas mixed response was defined as improvement of at least one evaluable organ with worsening in another organ as per International Blood and Marrow Transplantation Registry Severity Index Criteria grading system.
Complete Response No further treatment with RYONCIL Partial or Mixed Response* Repeat administration of RYONCIL once a week for additional 4 weeks (4 infusions total) No Response Consider alternative treatments Recurrence of GvHD after complete response Repeat administration of RYONCIL twice a week for an additional 4 consecutive weeks (8 infusions total) 2.2 Preparation and Administration Instructions
Receipt and Storage of RYONCIL
RYONCIL is shipped directly to the clinical facility in a liquid nitrogen dry shipper maintained at a temperature of ≤ -135°C.
RYONCIL must remain frozen at ≤ -135°C in liquid nitrogen vapor phase until thawed immediately prior to administration [see How Supplied/Storage and Handling (16)].
Preparation
RYONCIL and Plasma-Lyte® A should be prepared following aseptic technique in a Biological Safety Cabinet (BSC). Spray and wipe down the following materials with 70% alcohol prior to transferring them into the BSC preparation area. All materials should remain in the BSC preparation area unless discarded.
Prepare a sterile water bath to a minimum depth of 4 inches and warm to 37°C (± 2°C) at least 30 minutes prior for thawing.
Supplies needed for preparation of RYONCIL and Plasma-Lyte® A
- RYONCIL vials
- Plasma-Lyte® A
- Infusion bag
- Interlink blood bag spikes (2)
- Interlink threaded lock cannula (1 per syringe)
- 60 mL luer-lock syringe (1)
- 5 mL luer-lock syringe (1 per each thawed vial)
- 18-gauge needle (1 per each thawed vial)
Note: Use a 1mL syringe if volume to be removed from vial is less than 1mL - Airtight zip seal plastic bag(s) (1 per each vial for thaw)
- Water bath
- Alcohol wipes
Note: Plasma-Lyte® A may be substituted by Plasma-Lyte® 148 (pH 7.4 with no glucose).
Preparation of Plasma-Lyte® A
- 1- Insert the Interlink blood bag spike into the Plasma-Lyte® A bag.
- 2- Aseptically attach the threaded lock cannula to a 60 mL syringe.
- 3- Use an alcohol wipe to scrub the membrane of the Plasma-Lyte® A bag interlink spike injection site.
- 4- Attach the threaded lock cannula/syringe assembly to the Plasma-Lyte® A bag to the injection site.
- 5- Measure and withdraw 40 mL of Plasma-Lyte® A from the bag
Note: DO NOT remove the syringe containing Plasma-Lyte® A from the Plasma-Lyte® A bag. Set aside for later use.
Preparation of RYONCIL
- 1. Prior to RYONCIL thaw, verify that the pre-arranged time for the RYONCIL administration is still feasible. Patient infusion must occur within 5 hours from the start time of first vials of RYONCIL thaw.
- 2. Remove RYONCIL vial(s) from cryo-storage.
- 3.
Place vial(s) into an airtight zip seal plastic bag(s) and immerse closed bag(s) into the water bath (37ºC), maintaining the top closure above the water line. Use a separate zip seal plastic bag for each vial of RYONCIL.
Note: A maximum of 4 vials can be thawed in the water bath at the same time. - 4. To thaw, gently agitate the sealed zip seal bag(s) with the vial(s) for approximately 5 to 8 minutes to thaw.
- 5.
Remove vials from the water bath prior to the last visible crystal of ice melting.
Note: Do not exceed 15 minutes for each set of four vials. - 6. Inspect vial(s) while still in the bag(s) to identify there is no leakage of vial contents.
- 7. Remove the RYONCIL vial(s) from the sealed plastic bag(s).
- 8. Inspect for foreign particulate matter (FPM). If FPM is found via visual inspection – DO NOT USE! Retain the offending vial(s). Call the Mesoblast contact number 844-889-MESO (6376).
- 9. Remove the tab from the RYONCIL vial cap to expose the vial stopper and wipe the exposed surface of the stopper with one provided sterile alcohol wipe.
- 10. Promptly withdraw the required amount of RYONCIL (based on actual patient body weight) from the vial(s). One syringe and an 18-gauge needle per thawed vial is required.
- 11. Carefully remove the needle from the syringe containing RYONCIL.
- 12. Attach the threaded lock cannula to the syringe.
- 13. Retrieve the infusion bag.
- 14. Wipe the membrane of the infusion bag interlink spike injection site with sterile alcohol wipe.
- 15. Insert 1 interlink blood bag spike into the outermost port on the infusion bag (leave the middle port for the infusion line.)
- 16. Attach the syringe to the spike and transfer the RYONCIL into the infusion bag.
- 17. Remove the syringe with the threaded lock cannula and discard the syringe and the threaded lock cannula.
- 18. Repeat for each syringe until the required volume of RYONCIL is added to the infusion bag.
Transfer of Plasma-Lyte® A into Infusion Bag
- 1. Retrieve the bag and 60 mL syringe containing Plasma-Lyte® A.
- 2. Verify the syringe contains 40mL of Plasma-Lyte® A.
- 3. Aseptically remove the 60 mL syringe with the threaded lock cannula containing the 40mL Plasma-Lyte® from the bag of Plasma-Lyte® A.
- 4. Use alcohol wipes to clean the membrane of the infusion bag interlink spike injection site.
- 5. Aseptically attach the 60mL syringe containing the Plasma-Lyte® A to the spike.
- 6. Slowly transfer the 40mL Plasma-Lyte® A into the infusion bag.
- 7. Gently mix cells with the Plasma-Lyte® A.
- 8. Remove the syringe with the threaded lock cannula and discard the syringe and threaded lock cannula.
- 9. Label the bag according to Institutional Policy.
- 10. Transport infusion bag to patient infusion area.
Administration
Note: Patient infusion must occur within 5 hours from the start time of first vial(s) of RYONCIL thaw.
- Administer RYONCIL under the supervision of a qualified health professional experienced in the management of SR-aGvHD.
- Administer RYONCIL using an infusion pump.
- Use blood filter with a pore size of 40-260 microns for infusion of RYONCIL.
- Flush lines per institutional practice and/or policy for cellular infusions.
-
Pretreatment
Premedicate patients with corticosteroids and antihistamines 30-60 minutes prior to administration of RYONCIL to reduce the potential for infusion reactions. -
Infusion Rates
- For patients weighing 35 kg or more, infuse RYONCIL at a rate of no more than 6mL/ minute.
- For patients weighing less than 35 kg, infuse RYONCIL over the course of 60 minutes.
- Discard unused, thawed RYONCIL vials per institutional policy.
-
3 DOSAGE FORMS AND STRENGTHS
RYONCIL is available as a cell suspension for intravenous infusion in a target concentration of 6.68 X 106 MSCs per mL in 3.8 mL at cryopreservation contained in a 6 mL cryovial.
Each 6 mL cryovial contains approximately 25 x 106 MSCs.
Cryopreserved MSCs are combined with Plasma-Lyte® A for administration.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity and Acute Infusion Reactions
Hypersensitivity reactions including acute infusion reactions have occurred with RYONCIL administration [see Adverse Reactions (6.1)]. Serious hypersensitivity reactions, including anaphylaxis, may occur due to DMSO and trace amounts of porcine or bovine proteins. Signs and symptoms may include fever, dyspnea, and hypotension during or after RYONCIL infusion.
Premedicate patients with antihistamine and corticosteroids and monitor closely for signs and symptoms of hypersensitivity or acute infusion reactions.
If a hypersensitivity or infusion reaction occurs, interrupt RYONCIL infusion. Do not administer RYONCIL in patients who experience serious or life-threatening reactions.
5.2 Transmission of Infectious Agents
Transmission of infectious disease or agents may occur with RYONCIL administration because it contains cells from human donors and is manufactured using human, porcine and bovine-derived reagents. Donors are screened and tested for Human Immune-deficiency Virus 1 (HIV-1); Human Immune-deficiency Virus 2 (HIV-2); Hepatitis B Virus (HBV); Hepatitis C Virus (HCV); Human T-cell Leukemia-lymphoma Virus 1 (HTLV-1); Human T-cell Leukemia-lymphoma Virus 2 (HTLV-2); West Nile Virus (WNV); Cytomegalovirus (CMV); Epstein-Barr Virus (EBV); and Syphilis (Treponema pallidum). Only screening was performed for Creutzfeldt-Jakob disease (CJD) and communicable disease risks associated with xenotransplantation. RYONCIL cell banks are tested for human and animal viruses, retroviruses, bacteria, fungi, yeast, and mycoplasma. Human and animal-derived reagents are tested for human and animal viruses, bacteria, fungi, and mycoplasma before use. These measures do not eliminate the risk of transmitting these or other infectious diseases or agents.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The safety data described in this section reflect exposure to RYONCIL in 54 patients in Study MSB-GVHD001 for the treatment of SR-aGvHD. Patients received intravenous infusion of RYONCIL at a dosage of 2 x 106 MSCs/kg twice a week for four consecutive weeks, for a total of eight infusions. Patients with partial or mixed response at Day 28 received additional infusions of RYONCIL 2 x 106 MSCs/kg once a week for an additional four weeks [see Clinical Studies (14)]. The median number of doses administered were 10 (range 1 to 16), and the treatment was administered over a median of 43 days (range 1 to 104 days).
Serious adverse reactions occurred in 35 patients (65%) including pyrexia (n=5;9%), respiratory failure (n=5;9%), pneumatosis intestinalis (n=4;7%) and staphylococcal bacteremia (n=2;<5%). Eight patients had discontinuation of RYONCIL treatment due to the following: acute infusion reactions (n=3), hypotension (n=1), gastroenteritis (n=1), and death (n=3).
Table 2 summarizes most common adverse reactions that occurred in ≥10% patients in Study MSB-GVHD001.
Table 2: Adverse Reactions** Occurring in ≥10% of Patients in Study MSB-GVHD001 (N=54) a Based on National Cancer Institute Adverse Event Common Toxicity Criteria version 4.03 b No grade 4 or 5 adverse reactions occurred in the study *Is a composite that includes multiple related terms **Includes adverse reactions up to 100 days following RYONCIL treatment Adverse Reactions All Gradesa
n (%)Grade 3b
n (%)Viral infectious disorders* 30 (56) 8 (15) Bacterial infectious disorders* 24 (44) 10 (19) Infections - pathogen unspecified* 22 (41) 8 (15) Pyrexia 19 (35) 2 (4) Hemorrhage* 15 (28) 4 (7) Edema* 12 (22) 1 (2) Abdominal pain 11 (20) 4 (7) Hypertension 11 (20) 3 (6) Vomiting 10 (19) 3 (6) Arrhythmia* 9 (17) 2 (4) Diarrhea 9 (17) 1 (2) Rash* 9 (17) 0 (0) Arthralgia 8 (15) 0 (0) Fungal infectious disorders* 8 (15) 2 (4) Hypotension 8 (15) 2 (4) Cough 7 (13) 0 (0) Respiratory Failure 6 (11) 6 (11) Table 3 presents the most common grade 3 or 4 laboratory abnormalities that worsened from baseline in ≥10% of patients
Table 3: Grade 3 or 4 Laboratory Abnormalities that Worsened from Baseline in ≥ 10% of Patients in Study MSB-GVHD001 (N=54) Laboratory Parameter Grade 3 or highera
%a Based on National Cancer Institute Adverse Event Common Toxicity Criteria version 4.03 Gamma-glutamyl transferase increased 32 Thrombocytopenia 28 Blood bilirubin increased 11 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data for RYONCIL use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with RYONCIL to assess whether it can cause fetal harm when administered to a pregnant woman. It is not known if RYONCIL has the potential to be transferred to the fetus. Therefore, RYONCIL is not recommended for women who are pregnant. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 10-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of RYONCIL in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RYONCIL and any potential adverse effects on the breastfed infant from RYONCIL or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of RYONCIL for treatment of SR-GvHD have been established in pediatric patients 2 months of age and older. The use of RYONCIL in these age groups is supported by evidence from an adequate and well-controlled trial [see Adverse Reactions (6) and Clinical Studies (14)].
-
11 DESCRIPTION
RYONCIL is provided as a frozen cell suspension in a cryogenic vial. The active ingredient in RYONCIL is comprised of culture-expanded mesenchymal stromal cells (MSCs) isolated from the bone marrow of healthy human adult donors. Each cryovial contains nominally 25 x 106 MSCs in 3.8 mL (a target concentration 6.68 x 106 cells/mL) formulated in Plasma Lyte®-A (70% v/v), Human Serum Albumin (HSA) Solution (25%) (20% v/v) and Dimethyl sulfoxide (DMSO) (10% v/v). The product is thawed and combined with Plasma-Lyte® A prior to intravenous administration.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action for RYONCIL is not clear but may be related to immunomodulatory effects. Data from in vitro studies demonstrate that MSCs inhibit T cell activation as measured by proliferation and secretion of pro-inflammatory cytokines. Acute GvHD occurs when alloreactive donor-derived T cells within the donated tissue (graft) trigger an immunological response, and alloreactive donor-derived T cells play a role in mediating the systemic inflammation, cytotoxicity and potential end organ damage associated with aGvHD.
12.2 Pharmacodynamics
Human pharmacodynamic data were obtained from analysis of blood samples in pediatric subjects with SR-aGvHD (n=40; age range 0.6-17 years) following treatment with RYONCIL at a dose of 2x106 cells/kg. At Baseline, elevated levels of tumor necrosis factor receptor type I (TNFR1) and suppressor of tumorigenicity 2 (ST2) were observed consistent with the inflammatory state of aGvHD. Treatment with RYONCIL reduced the levels of TNFR1 and ST2 by 79% and 75%, respectively, at Day 180 as compared to baseline values. Further, the circulating levels of CD3+CD4+CD25+HLA-DR+ T cells, which represent activated T cells, were reduced by 64% at Day 180 following treatment with RYONCIL as compared to the baseline values.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of RYONCIL was evaluated in a multicenter, prospective, single-arm study (MSB-GVHD 001; NCT02336230). The study enrolled pediatric patients with SR-GvHD Grade B to D (excluding Grade B skin alone) as per International Blood and Marrow Transplantation Registry Severity Index Criteria (IBMTR) after receiving allogeneic hematopoietic stem cell transplantation (HSCT). SR-aGvHD was defined as aGvHD that progressed within 3 days or did not improve within 7 consecutive days of treatment with methylprednisolone 2 mg/kg/day or equivalent. Patients who received a second line therapy for aGvHD prior to screening were excluded.
RYONCIL was administered by intravenous infusion at a dosage of 2 x 106 MSCs/kg twice a week for four consecutive weeks, for a total of eight infusions. Patients with partial or mixed response at Day 28 received additional infusions of RYONCIL 2 x 106 MSCs/kg once a week for an additional four consecutive weeks. Patients with complete response at Day 28 who experienced recurrence of aGvHD received infusions of RYONCIL 2 x 106 MSCs/kg twice a week for an additional four consecutive weeks.
A total of 55 patients were enrolled, and 54 were treated with RYONCIL. Among the treated patients (n=54), the demographic characteristics were as follows: median age was 7 years (range: 7 months to 17 years); 36% were females; 56% were White, 19% reported “other” race, 15% were Black, 6% were Asian, 6% American Indian or Alaska Native, 33% were Hispanic and 65% were non-Hispanic. Among enrolled patients, hematologic malignancies (67%) and non-malignant diseases (33%) were the underlying reasons for allogenic HSCT. SR-GvHD severity was as follows at baseline: Grade B (11%), Grade C (43%), Grade D (46%). Organ involvement at baseline were as follows: skin alone (26%), lower gastrointestinal tract only (39%), multi-organ involvement (35%). The median duration of prior corticosteroid treatment at baseline was 8 days (range: 2 to 46 days).
The main efficacy outcome measures were Day-28 overall response rate (complete response rate and partial response rate) and the duration of response.
The efficacy results are summarized in Table 4.
Table 4: Efficacy Results for Study MSB-GVHD001 Response at Day 28 N=54 CI=confidence interval a Two-sided exact binomial confidence interval at the 95% level b Duration of response was calculated from Day-28 response to either progression (worsening by one stage in any organ without improvement in other organs in comparison to prior response assessment), new systemic therapy for aGvHD or death from any cause. c Complete response was defined as resolution of aGvHD in all involved organs as per IBMTR grading system. d Partial response was defined as organ improvement of at least one stage without worsening of any other organ as per IBMTR grading system. Overall Response Rate
n (%)
95% CIa
38 (70%)
56.4, 82.0
Durationb (days)
Median (range)
54 (7, 159+)Complete Response Ratec
n (%)
95% CIa
16 (30%)
18.0, 43.6Partial Response Rated
n (%)
95% CIa
22 (41%)
27.6, 55.0Overall response rate at Day 28 by baseline disease severity is as follows: Grade B (3/6; 50%), Grade C (16/23; 70%), and Grade D (19/25; 76%).
Among the 38 responders, the median time from Day-28 response to either death or need for systemic therapy for aGvHD (non-RYONCIL systemic therapy for aGvHD or increase in the dose of corticosteroids to methylprednisolone 2 mg/kg or equivalent) was 111.5 days (range 9, 182+).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
RYONCIL is supplied as a sterile, cryopreserved cell suspension of ex-vivo culture-expanded allogeneic bone marrow-derived mesenchymal stromal cells (MSC) in vials.
RYONCIL is provided as a customized kit to meet dosing requirements for a single dose for each patient [see Dosage and Administration (2.1)], with each kit containing:
- Cartons containing sufficient RYONCIL for one infusion based on the patient weight (see Table 5)
- Sufficient alcohol wipes for preparation of the RYONCIL infusions.
Kit sizes and National Drug Codes (NDC) are provided in Table 5.
Table 5: RYONCIL Kit Sizes Patient weight (kg) Kit contents (single infusion) Number of kits needed for: 4-vial cartons 1-vial cartons Total cartons Number of alcohol wipes NDC Number Initial course 2nd course Relapse after CR <12.5 0 1 1 1 73648-111-01 8 4 8 12.5-<25 0 2 2 2 73648-112-02 25-<37.5 0 3 3 3 73648-113-03 37.5-<50 1 0 1 4 73648-114-01 50-<62.5 1 1 2 5 73648-115-02 62.5-<75 1 2 3 6 73648-116-03 75-<87.5 1 3 4 7 73648-117-04 87.5-<100 2 0 2 8 73648-118-02 100-<112.5 2 1 3 9 73648-119-03 112.5-<125 2 2 4 10 73648-120-04 125-<137.5 2 3 5 11 73648-121-05 137.5-<150 3 0 3 12 73648-122-03 RYONCIL is shipped to the clinical facility in a liquid nitrogen dry shipper maintained at a temperature of ≤ -135°C.
Storage conditions: RYONCIL must remain frozen at ≤ -135°C in liquid nitrogen vapor phase until thawed immediately prior to administration.
Handling: Restrict preparation and administration of RYONCIL to a medical facility in which the medical personnel are trained in aseptic technique.
Disposal: Dispose empty or partially used RYONCIL vials according to the institutional guidelines for disposal of biohazard materials.
Do not save thawed RYONCIL vials for future use.
-
17 PATIENT COUNSELING INFORMATION
Discuss the following with patients and/or caregivers.
- Hypersensitivity and Acute infusion reactions: Inform patients and/or caregivers that hypersensitivity and acute infusion reactions due to the presence of porcine and bovine protein, and DMSO may occur with RYONCIL infusion. Advise patients and/or caregivers to seek immediate medical evaluation if any signs and symptoms of hypersensitivity or acute infusion reactions occur, such as fever, rash or hives, low blood pressure, breathing problems [see Warnings and Precautions (5.1].
- Transmission of Infectious Agents: Inform patients and/or caregivers about the possible risk of transmission of infectious agents with RYONCIL administration [see Warnings and Precautions (5.2)].
- Ectopic Tissue Formation: Inform patients and/or caregivers about the possible risk of ectopic tissue formation with RYONCIL administration [see Warnings and Precautions (5.3)].
Manufactured for:
Mesoblast, Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
US License number: 2140RYONCIL is a registered trademark of Mesoblast. All rights reserved.
©2024 Mesoblast, Inc. All rights Reserved
V 3 - PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
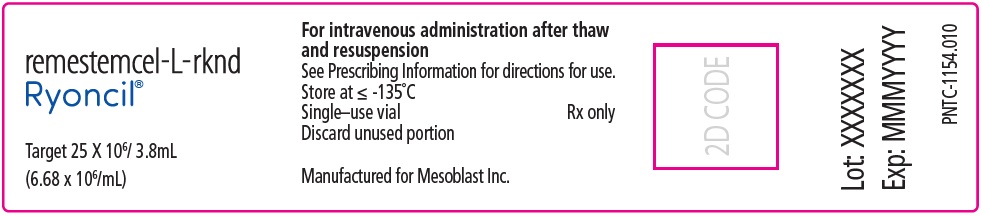
NDC 73648-154-01
remestemcel-L-rknd
Ryoncil®
Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)
Single–use vial. Discard unused portion.For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.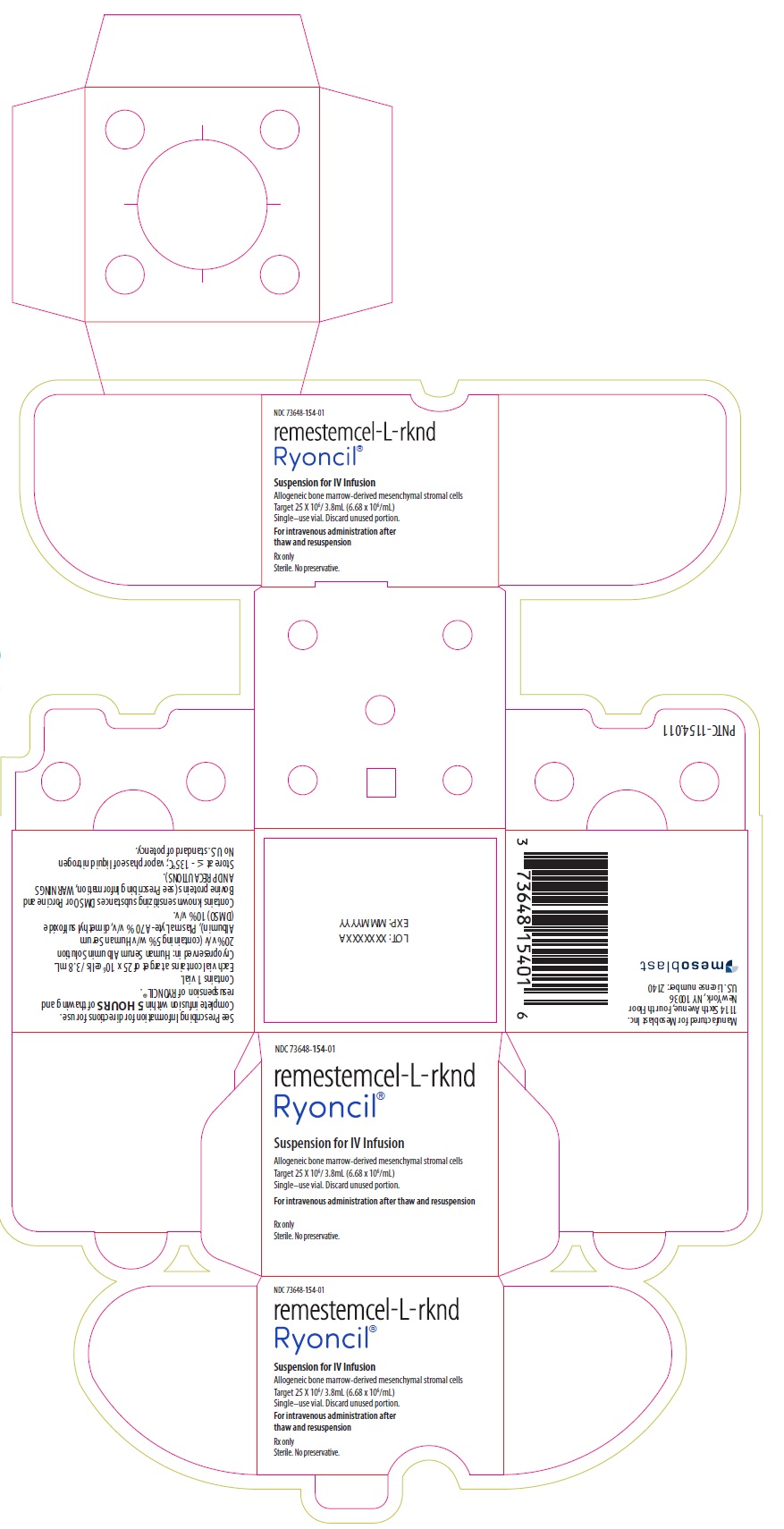
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 73648-154-04
remestemcel-L-rknd
Ryoncil®
Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)
Single–use vial. Discard unused portion.For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.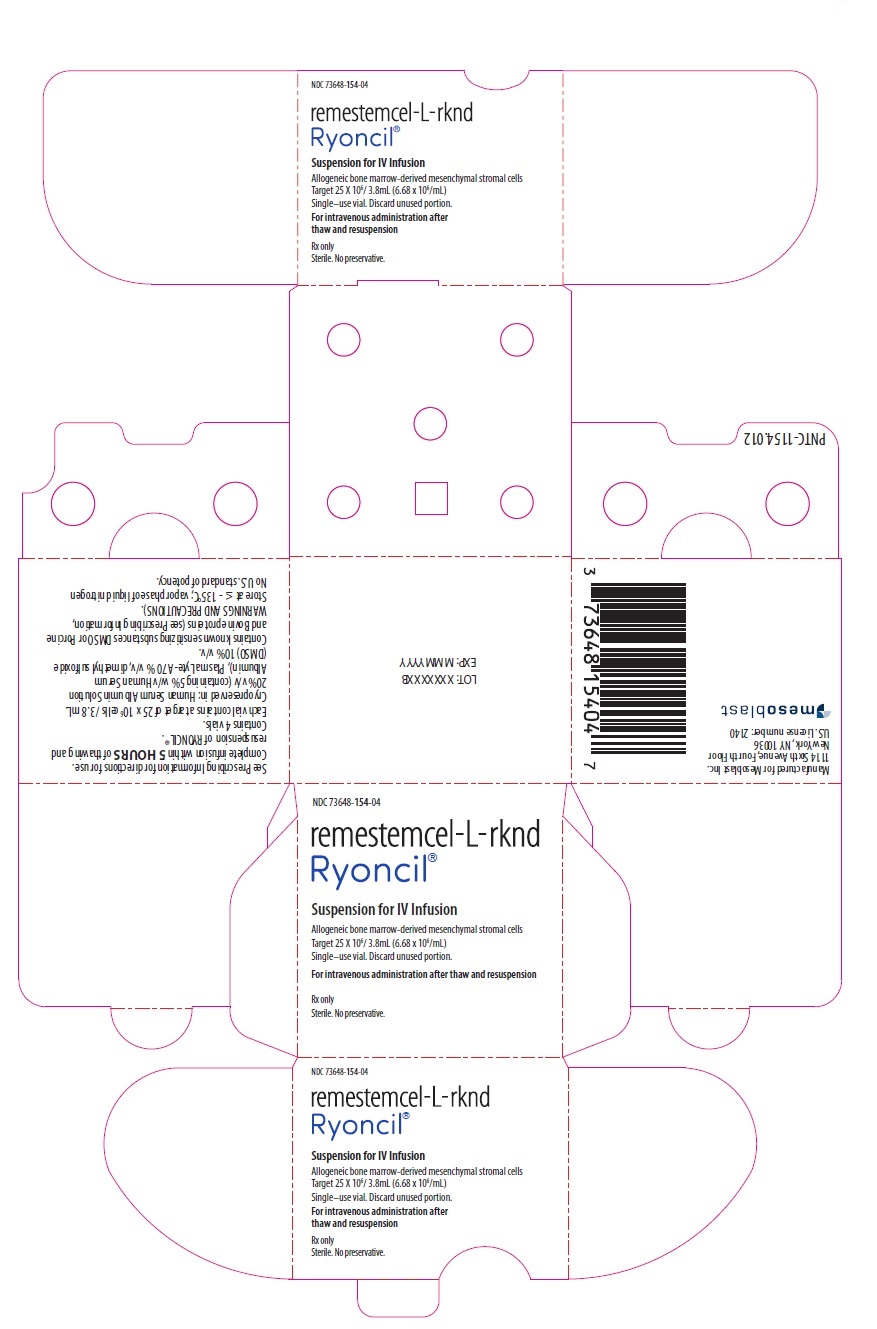
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-111-01
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
<12.5 kgKit Contents:
1 X single vial carton
1 Alcohol WipeSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.024

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-112-02
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
12.5 kg – <25 kgKit Contents:
2 X single vial cartons
2 Alcohol WipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.025

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-113-03
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
25 kg – <37.5 kgKit Contents:
3 X single vial cartons
3 Alcohol WipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.026

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-114-01
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
37.5 kg – <50 kgKit Contents:
1 X 4-vial carton
4 Alcohol WipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.027

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-115-02
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
50 kg – <62.5 kgKit Contents:
1 X single vial carton
1 X 4-vial carton
5 Alcohol WipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.028

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-116-03
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
62.5 kg – <75 kgKit Contents:
2 X single vial cartons
1 X 4-vial carton
6 Alcohol WipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.029

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-117-04
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
75 kg – <87.5 kgKit Contents:
3 X single vial cartons
1 X 4-vial carton
7 Alcohol WipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.030

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-118-02
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
87.5 kg – <100 kgKit Contents:
2 X 4-vial cartons
8 Alcohol WipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.031

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-119-03
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
100 - < 112.5 kgKit Contents:
1 x Single vial carton
2 x 4-vial cartons
9 Alcohol wipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.038

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-120-04
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
112.5 - < 125 kgKit Contents:
2 x Single vial carton
2 x 4-vial cartons
10 Alcohol wipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.039

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-121-05
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
125 - < 137.5 kgKit Contents:
3 x Single vial carton
2 x 4-vial cartons
11 Alcohol wipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.040

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 73648-122-03
remestemcel-L-rknd
Ryoncil®Suspension for IV Infusion
Allogeneic bone marrow-derived mesenchymal stromal cells
Target 25 X 106/ 3.8mL (6.68 x 106/mL)Kit for Patient Weight:
137.5 - < 150 kgKit Contents:
3 x 4-vial cartons
12 Alcohol wipesSingle–use vial. Discard unused portion.
For intravenous administration after thaw and resuspension
Rx only
Sterile. No preservative.
See Prescribing Information for directions for use.
Complete infusion within 5 HOURS of thawing and resuspension
of RYONCIL®.
Each vial contains a target of 25 x 106 cells / 3.8 mL.
Cryopreserved in: Human Serum Albumin Solution 20% v/v
(containing 5% w/v Human Serum Albumin), Plasma Lyte-A 70 %
v/v, dimethyl sulfoxide (DMSO) 10% v/v.
Contains known sensitizing substances DMSO or Porcine and
Bovine proteins (see Prescribing Information, WARNINGS AND
PRECAUTIONS).
Store at ≤- 135°C; vapor phase of liquid nitrogen
No U.S. standard of potency.Manufactured for Mesoblast Inc.
1114 Sixth Avenue, Fourth Floor
New York, NY 10036
U.S. License number: 2140GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXX
LOT: XXXXXXX
EXP: MMMYYYYPNTC-1154.041

-
INGREDIENTS AND APPEARANCE
RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-111 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-111-01 1 in 1 CARTON; Type 0: Not a Combination Product 03/17/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, PLASTIC 3.8 mL Part 2 1 POUCH 1 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 03/17/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-112 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-112-02 1 in 1 CARTON; Type 0: Not a Combination Product 03/17/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, PLASTIC 7.6 mL Part 2 2 POUCH 2 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 1 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 03/17/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-113 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-113-03 1 in 1 CARTON; Type 0: Not a Combination Product 03/17/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 3 VIAL, PLASTIC 11.4 mL Part 2 3 POUCH 3 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 1 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 3 1 in 1 CARTON 3 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 03/17/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-114 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-114-01 1 in 1 CARTON; Type 0: Not a Combination Product 03/17/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 4 VIAL, PLASTIC 15.2 mL Part 2 4 POUCH 4 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 03/17/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-115 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-115-02 1 in 1 CARTON; Type 0: Not a Combination Product 03/17/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 5 VIAL, PLASTIC 19 mL Part 2 5 POUCH 5 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 4 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 03/17/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-116 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-116-03 1 in 1 CARTON; Type 0: Not a Combination Product 03/17/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 VIAL, PLASTIC 22.8 mL Part 2 6 POUCH 6 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 1 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 3 4 in 1 CARTON 3 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 03/17/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-117 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-117-04 1 in 1 CARTON; Type 0: Not a Combination Product 03/17/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 7 VIAL, PLASTIC 26.6 mL Part 2 7 POUCH 7 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 1 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 3 1 in 1 CARTON 3 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 4 4 in 1 CARTON 4 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 03/17/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-118 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-118-02 1 in 1 CARTON; Type 0: Not a Combination Product 03/17/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 8 VIAL, PLASTIC 30.4 mL Part 2 8 POUCH 8 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 4 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 03/17/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-119 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-119-03 1 in 1 CARTON; Type 0: Not a Combination Product 09/28/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 9 VIAL, PLASTIC 34.2 mL Part 2 9 POUCH 9 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 4 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 3 4 in 1 CARTON 3 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 09/28/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-120 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-120-04 1 in 1 CARTON; Type 0: Not a Combination Product 09/28/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL, PLASTIC 38 mL Part 2 10 POUCH 10 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 1 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 3 4 in 1 CARTON 3 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 4 4 in 1 CARTON 4 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 09/28/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-121 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-121-05 1 in 1 CARTON; Type 0: Not a Combination Product 09/28/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 11 VIAL, PLASTIC 41.8 mL Part 2 11 POUCH 11 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 1 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 3 1 in 1 CARTON 3 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 4 4 in 1 CARTON 4 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 5 4 in 1 CARTON 5 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 09/28/2025 RYONCIL
remestemcel-l-rknd kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73648-122 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73648-122-03 1 in 1 CARTON; Type 0: Not a Combination Product 09/28/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 12 VIAL, PLASTIC 45.6 mL Part 2 12 POUCH 12 mL Part 1 of 2 RYONCIL
remestemcel-l-rknd suspensionProduct Information Item Code (Source) NDC: 73648-154 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS (UNII: OEN4982XNW) (ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS - UNII:OEN4982XNW) ALLOGENIC BONE-MARROW-DERIVED MESENCHYMAL STEM CELLS 6680000 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength PLASMALYTE A (UNII: MZD2VV6EW6) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALBUMIN HUMAN (UNII: ZIF514RVZR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4 in 1 CARTON 1 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 2 4 in 1 CARTON 2 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product 3 4 in 1 CARTON 3 3.8 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 Part 2 of 2 ALCOHOL SWAB
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 73648-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125706 09/28/2025 Labeler - Mesoblast (616697426) Establishment Name Address ID/FEI Business Operations Mesoblast, Inc. 616697426 manufacture(73648-154, 73648-111, 73648-112, 73648-113, 73648-114, 73648-115, 73648-116, 73648-117, 73648-118, 73648-119, 73648-120, 73648-121, 73648-122) Establishment Name Address ID/FEI Business Operations LONZA BIOLOGICS TUAS PTE. LTD. 936939342 manufacture(73648-154) , analysis(73648-154) , label(73648-154) , pack(73648-154) Establishment Name Address ID/FEI Business Operations Integrated Commercialization Solutions, LLC 832820588 pack(73648-154, 73648-111, 73648-112, 73648-113, 73648-114, 73648-115, 73648-116, 73648-117, 73648-118, 73648-119, 73648-120, 73648-121, 73648-122) Establishment Name Address ID/FEI Business Operations WuXi Advanced Therapies Inc. 117556312 analysis(73648-154)
Trademark Results [RYONCIL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RYONCIL 88978590 not registered Live/Pending |
Mesoblast International SÃ rl 2019-02-26 |
 RYONCIL 88317318 not registered Live/Pending |
Mesoblast International SÃ rl 2019-02-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.