innisfree Gentle Care for Sensitive Skin Sunscreen
innisfree Gentle Care for Sensitive Skin Sunscreen by
Drug Labeling and Warnings
innisfree Gentle Care for Sensitive Skin Sunscreen by is a Otc medication manufactured, distributed, or labeled by Innisfree Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
INNISFREE GENTLE CARE FOR SENSITIVE SKIN SUNSCREEN- titanium dioxide lotion lotion
Innisfree Corporation
----------
innisfree Gentle Care for Sensitive Skin Sunscreen
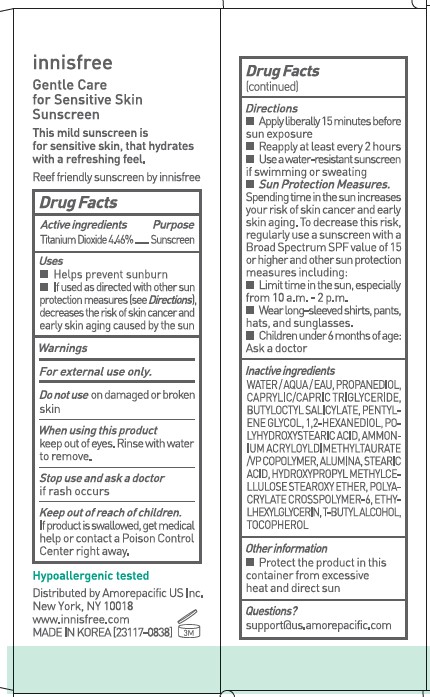
USES
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
DIRECTIONS
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including :
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age : Ask a doctor.
INACTIVE INGREDIENTS
WATER / AQUA / EAU, PROPANEDIOL, CAPRYLIC/CAPRIC TRIGLYCERIDE, BUTYLOCTYL SALICYLATE, PENTYLENE GLYCOL, 1,2-HEXANEDIOL, POLYHYDROXYSTEARIC ACID, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, ALUMINA, STEARIC ACID, HYDROXYPROPYL METHYLCELLULOSE STEAROXY ETHER, POLYACRYLATE CROSSPOLYMER-6, ETHYLHEXYLGLYCERIN, T-BUTYL ALCOHOL, TOCOPHEROL
| INNISFREE GENTLE CARE FOR SENSITIVE SKIN SUNSCREEN
titanium dioxide lotion lotion |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Innisfree Corporation (557822425) |
Revised: 11/2023
Document Id: 0b52ec38-ed7a-5edd-e063-6294a90a0278
Set id: a51530af-3f54-833c-e053-2a95a90a1629
Version: 3
Effective Time: 20231129
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.