REOPRO- abciximab injection, solution
REOPRO by
Drug Labeling and Warnings
REOPRO by is a Prescription medication manufactured, distributed, or labeled by Janssen Biotech, Inc., Janssen Biologics B.V., Hospira Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Abciximab, ReoPro®, is the Fab fragment of the chimeric human-murine monoclonal antibody 7E3. Abciximab binds to the glycoprotein (GP) IIb/IIIa receptor of human platelets and inhibits platelet aggregation. Abciximab also binds to the vitronectin (αvβ3) receptor found on platelets and vessel wall endothelial and smooth muscle cells.
The chimeric 7E3 antibody is produced by continuous perfusion in mammalian cell culture. The 47,615 dalton Fab fragment is purified from cell culture supernatant by a series of steps involving specific viral inactivation and removal procedures, digestion with papain and column chromatography.
ReoPro® is a clear, colorless, sterile, non-pyrogenic solution for intravenous (IV) use. Each single-dose vial contains 2 mg/mL of Abciximab in a buffered solution (pH 7.2) of 0.01 M sodium phosphate, 0.15 M sodium chloride and 0.001% polysorbate 80 in Water for Injection. No preservatives are added.
-
CLINICAL PHARMACOLOGY:
General- Abciximab binds to the intact platelet GPIIb/IIIa receptor, which is a member of the integrin family of adhesion receptors and the major platelet surface receptor involved in platelet aggregation. Abciximab inhibits platelet aggregation by preventing the binding of fibrinogen, von Willebrand factor, and other adhesive molecules to GPIIb/IIIa receptor sites on activated platelets. The mechanism of action is thought to involve steric hindrance and/or conformational effects to block access of large molecules to the receptor rather than direct interaction with the RGD (arginine-glycine-aspartic acid) binding site of GPIIb/IIIa.
Abciximab binds with similar affinity to the vitronectin receptor, also known as the αvβ3 integrin. The vitronectin receptor mediates the procoagulant properties of platelets and the proliferative properties of vascular endothelial and smooth muscle cells. In in vitro studies using a model cell line derived from melanoma cells, Abciximab blocked αvβ3-mediated effects including cell adhesion (IC50 = 0.34 μg/mL). At concentrations which, in vitro, provide > 80% GPIIb/IIIa receptor blockade, but above the in vivo therapeutic range, Abciximab more effectively blocked the burst of thrombin generation that followed platelet activation than select comparator antibodies which inhibit GPIIb/IIIa alone (1). The relationship of these in vitro data to clinical efficacy is unknown.
Abciximab also binds to the activated Mac-1 receptor on monocytes and neutrophils (2). In in vitro studies, Abciximab and 7E3 IgG blocked Mac-1 receptor function as evidenced by inhibition of monocyte adhesion (3). In addition, the degree of activated Mac-1 expression on circulating leukocytes and the numbers of circulating leukocyte-platelet complexes has been shown to be reduced in patients treated with Abciximab compared to control patients (4). The relationship of these in vitro data to clinical efficacy is uncertain.
Pre-clinical experience- Maximal inhibition of platelet aggregation was observed when ≥ 80% of GPIIb/IIIa receptors were blocked by Abciximab. In non-human primates, Abciximab bolus doses of 0.25 mg/kg generally achieved a blockade of at least 80% of platelet receptors and fully inhibited platelet aggregation. Inhibition of platelet function was temporary following a bolus dose, but receptor blockade could be sustained at ≥ 80% by continuous intravenous infusion. The inhibitory effects of Abciximab were substantially reversed by the transfusion of platelets in monkeys. The antithrombotic efficacy of prototype antibodies [murine 7E3 Fab and F(ab´)2] and Abciximab was evaluated in dog, monkey and baboon models of coronary, carotid, and femoral artery thrombosis. Doses of the murine version of 7E3 or Abciximab sufficient to produce high-grade (≥ 80%) GPIIb/IIIa receptor blockade prevented acute thrombosis and yielded lower rates of thrombosis compared with aspirin and/or heparin.
Pharmacokinetics- Following intravenous bolus administration, free plasma concentrations of Abciximab decrease rapidly with an initial half-life of less than 10 minutes and a second phase half-life of about 30 minutes, probably related to rapid binding to the platelet GPIIb/IIIa receptors. Platelet function generally recovers over the course of 48 hours (5,6), although Abciximab remains in the circulation for 15 days or more in a platelet-bound state. Intravenous administration of a 0.25 mg/kg bolus dose of Abciximab followed by continuous infusion of 10 μg/min (or a weight-adjusted infusion of 0.125 μg/kg/min to a maximum of 10 μg/min) produces approximately constant free plasma concentrations throughout the infusion. At the termination of the infusion period, free plasma concentrations fall rapidly for approximately six hours then decline at a slower rate.
Pharmacodynamics- Intravenous administration in humans of single bolus doses of Abciximab from 0.15 mg/kg to 0.30 mg/kg produced rapid dose-dependent inhibition of platelet function as measured by ex vivo platelet aggregation in response to adenosine diphosphate (ADP) or by prolongation of bleeding time. At the two highest doses (0.25 and 0.30 mg/kg) at two hours post injection (the first time point evaluated), over 80% of the GPIIb/IIIa receptors were blocked and platelet aggregation in response to 20 μM ADP was almost abolished. The median bleeding time increased to over 30 minutes at both doses compared with a baseline value of approximately five minutes.
Intravenous administration in humans of a single bolus dose of 0.25 mg/kg followed by a continuous infusion of 10 μg/min for periods of 12 to 96 hours produced sustained high-grade GPIIb/IIIa receptor blockade (≥ 80%) and inhibition of platelet function (ex vivo platelet aggregation in response to 5 μM or 20 μM ADP less than 20% of baseline and bleeding time greater than 30 minutes) for the duration of the infusion in most patients. Similar results were obtained when a weight-adjusted infusion dose (0.125 μg/kg/min to a maximum of 10 μg/min) was used in patients weighing up to 80 kg. Results in patients who received the 0.25 mg/kg bolus followed by a 5 μg/min infusion for 24 hours showed a similar initial receptor blockade and inhibition of platelet aggregation, but the response was not maintained throughout the infusion period. The onset of Abciximab-mediated platelet inhibition following a 0.25 mg/kg bolus and 0.125 μg/kg/min infusion was rapid and platelet aggregation was reduced to less than 20% of baseline in 8 of 10 patients at 10 minutes after treatment initiation.
Low levels of GPIIb/IIIa receptor blockade are present for more than 10 days following cessation of the infusion. After discontinuation of Abciximab infusion, platelet function returns gradually to normal. Bleeding time returned to ≤ 12 minutes within 12 hours following the end of infusion in 15 of 20 patients (75%), and within 24 hours in 18 of 20 patients (90%). Ex vivo platelet aggregation in response to 5 μM ADP returned to ≥ 50% of baseline within 24 hours following the end of infusion in 11 of 32 patients (34%) and within 48 hours in 23 of 32 patients (72%). In response to 20 μM ADP, ex vivo platelet aggregation returned to ≥ 50% of baseline within 24 hours in 20 of 32 patients (62%) and within 48 hours in 28 of 32 patients (88%).
-
CLINICAL STUDIES:
Abciximab has been studied in four Phase 3 clinical trials, all of which evaluated the effect of Abciximab in patients undergoing percutaneous coronary intervention (PCI): in patients at high risk for abrupt closure of the treated coronary vessel (EPIC), in a broader group of patients (EPILOG), in unstable angina patients not responding to conventional medical therapy (CAPTURE), and in patients suitable for either conventional angioplasty/atherectomy or primary stent implantation (EPILOG Stent; EPISTENT). Percutaneous intervention included balloon angioplasty, atherectomy, or stent placement. All trials involved the use of various, concomitant heparin dose regimens and, unless contraindicated, aspirin (325 mg) was administered orally two hours prior to the planned procedure and then once daily.
EPIC was a multicenter, double-blind, placebo-controlled trial of Abciximab in patients undergoing percutaneous transluminal coronary angioplasty or atherectomy (PTCA) who were at high risk for abrupt closure of the treated coronary vessel (7). Patients were allocated to treatment with: 1) Abciximab bolus plus infusion for 12 hours; 2) Abciximab bolus plus placebo infusion, or; 3) placebo bolus plus infusion. All patients received concomitant heparin (10,000 to 12,000 U bolus followed by an infusion for 12 hours).
The primary endpoint was the composite of death, myocardial infarction (MI), or urgent intervention for recurrent ischemia within 30 days of randomization. The primary endpoint event rates in the Abciximab bolus plus infusion group were reduced mostly in the first 48 hours and this benefit was sustained through 30 days (7), 6 months (8), and three years (9).
EPILOG was a randomized, double-blind, multicenter, placebo-controlled trial which evaluated Abciximab in a broad population of patients undergoing PCI (excluding patients with myocardial infarction and unstable angina meeting the EPIC high risk criteria) (10). Study procedures emphasized discontinuation of heparin after the procedure with early femoral arterial sheath removal and careful access site management (see PRECAUTIONS). EPILOG was a three-arm trial comparing Abciximab plus standard-dose heparin, Abciximab plus low-dose heparin, and placebo plus standard-dose heparin. Abciximab and heparin infusions were weight-adjusted in all arms. The Abciximab bolus plus infusion regimen was: 0.25 mg/kg bolus followed by a 0.125 μg/kg/min infusion (to a maximum of 10 μg/min) for 12 hours. The heparin regimen was either a standard-dose regimen (initial 100 U/kg bolus, target ACT ≥ 300 seconds) or a low-dose regimen (initial 70 U/kg bolus, target ACT ≥ 200 seconds).
The primary endpoint of the EPILOG trial was the composite of death or MI occurring within 30 days of PCI. The composite of death, MI, or urgent intervention was an important secondary endpoint. The endpoint events in the Abciximab treatment group were reduced mostly in the first 48 hours and this benefit was sustained through 30 days and six months (10) and one year (11). The (Kaplan-Meier) endpoint event rates at 30 days are shown in Table 1.
Table 1 ENDPOINT EVENT RATES AT 30 DAYS - EPILOG TRIAL
Placebo +
Standard Dose Heparin
(n=939)Abciximab +
Standard Dose Heparin
(n=918)Abciximab +
Low Dose
Heparin
(n=935)
Number of Patients (%)
a Patients who experienced more than one event in the first 30 days are counted only once.
b Patients are counted only once under the most serious component (death > acute MI >
urgent intervention).Death or MIa 85 (9.1) 38 (4.2) 35 (3.8) p-value vs. placebo <0.001 <0.001 Death, MI, or urgent interventiona 109 (11.7) 49 (5.4) 48 (5.2) p-value vs. placebo <0.001 <0.001 Components of Composite Endpointsb Death 7 (0.8) 4 (0.4) 3 (0.3) Acute myocardial infarctions in surviving patients 78 (8.4) 34 (3.7) 32 (3.4) Urgent interventions in surviving patients without an acute myocardial infarction 24 (2.6) 11 (1.2) 13 (1.4) At the six-month follow up visit, the event rate for death, MI, or repeat (urgent or non-urgent) intervention remained lower in the Abciximab treatment arms (22.3% and 22.8%, respectively, for the standard- and low-dose heparin arms) than in the placebo arm (25.8%) and the event rate for death, MI, or urgent intervention was substantially lower in the Abciximab treatment arms (8.3% and 8.4%, respectively, for the standard- and low-dose heparin arms) than in the placebo arm (14.7%). The treatment associated effects continued to persist at the one-year follow up visit. The proportionate reductions in endpoint event rates were similar irrespective of the type of coronary intervention used (balloon angioplasty, atherectomy, or stent placement). Risk assessment using the American College of Cardiology/American Heart Association clinical/morphological criteria had large inter-observer variability. Consequently, a low risk subgroup could not be reproducibly identified in which to evaluate efficacy.
The EPISTENT trial was a randomized, multicenter trial evaluating three different treatment strategies in patients undergoing PCI: conventional PTCA with Abciximab plus low-dose heparin, primary intracoronary stent implantation with Abciximab plus low-dose heparin, and primary intracoronary stent implantation with placebo plus standard-dose heparin (12). The heparin dose was weight-adjusted in all arms. The JJIS Palmaz-Schatz stent was used in over 90% of the patients receiving stents. The two stent arms were blinded with respect to study agent (Abciximab or placebo) and heparin dose; the PCI arm with Abciximab was open-label. The Abciximab bolus plus infusion regimen was the same as that used in the EPILOG trial. The standard-dose and low-dose heparin regimens were the same as those used in the EPILOG trial. All patients were to receive aspirin; ticlopidine, if given, was to be started prior to study agent. Patient and access site management guidelines were the same as those for EPILOG, including a strong recommendation for early sheath removal.
The results demonstrated benefit in both Abciximab arms (i.e., with and without stents) compared with stenting alone on the composite of death, MI, or urgent intervention (repeat PCI or CABG) within 30 days of PCI (12). The (Kaplan-Meier) endpoint event rates at 30 days are shown in Table 2.
Table 2 PRIMARY ENDPOINT EVENT RATE AT 30 DAYS - EPISTENT TRIAL Placebo + Stent
(n=809)Abciximab + Stent
(n=794)Abciximab + PTCA
(n=796)Number of Patients (%) a Patients who experienced more than one event in the first 30 days are counted only once.
b Patients are counted only once under the most serious component (death > acute MI > urgent intervention).
Death, MI, or urgent interventiona 87 (10.8%) 42 (5.3%) 55 (6.9%) p-value vs. placebo <0.001 0.007 Components of Composite Endpointb Death 5 (0.6%) 2 (0.3%) 6 (0.8%) Acute myocardial infarctions in surviving patients 77 (9.6%) 35 (4.4%) 40 (5.0%) Urgent interventions in surviving patients without an acute myocardial infarction 5 (0.6%) 5 (0.6%) 9 (1.1%) This benefit was maintained at 6 months: 12.1% of patients in the placebo/stent group experienced death, MI, or urgent revascularization compared with 6.4% of patients in the Abciximab/stent group (p<0.001 vs placebo/stent) and 9.2% in the Abciximab/PTCA group (p=0.051 vs placebo/stent). At 6 months, a reduction in the composite of death, MI, or all repeat (urgent or non-urgent) intervention was observed in the Abciximab/stent group compared with the placebo/stent group (15.4% vs 20.4%, p=0.006); the rate of this composite endpoint was similar in the Abciximab/PTCA and placebo/stent groups (22.4% vs 20.4%, p=0.467). (13)
CAPTURE was a randomized, double-blind, multicenter, placebo-controlled trial of the use of Abciximab in unstable angina patients not responding to conventional medical therapy for whom PCI was planned, but not immediately performed (14). The CAPTURE trial involved the administration of placebo or Abciximab starting 18 to 24 hours prior to PCI and continuing until one hour after completion of the intervention.
Patients were assessed as having unstable angina not responding to conventional medical therapy if they had at least one episode of myocardial ischemia despite bed rest and at least two hours of therapy with intravenous heparin and oral or intravenous nitrates. These patients were enrolled into the CAPTURE trial, if during a screening angiogram, they were determined to have a coronary lesion amenable to PCI. Patients received a bolus dose and intravenous infusion of placebo or Abciximab for 18 to 24 hours. At the end of the infusion period, the intervention was performed. The Abciximab or placebo infusion was discontinued one hour following the intervention. Patients were treated with intravenous heparin and oral or intravenous nitrates throughout the 18- to 24-hour Abciximab infusion period prior to the PCI.
The Abciximab dose was a 0.25 mg/kg bolus followed by a continuous infusion at a rate of 10 μg/min. The CAPTURE trial incorporated weight adjustment of the standard heparin dose only during the performance of the intervention, but did not investigate the effect of a lower heparin dose, and arterial sheaths were left in place for approximately 40 hours. The primary endpoint of the CAPTURE trial was the occurrence of any of the following events within 30 days of PCI: death, MI, or urgent intervention. The 30-day (Kaplan-Meier) primary endpoint event rates are shown in Table 3.
Table 3 PRIMARY ENDPOINT EVENT RATE AT 30 DAYS – CAPTURE TRIAL Placebo
(n=635)Abciximab
(n=630)Number of Patients (%) a Patients who experienced more than one event in the first 30 days are counted only once. Urgent interventions included any unplanned PCI after the planned intervention, as well as any stent placement for immediate patency and any unplanned CABG or use of an intra-aortic balloon pump.
b Patients are counted only once under the most serious component (death > acute MI > urgent intervention).
Death, MI, or urgent interventiona 101 (15.9) 71 (11.3) p-value vs. placebo 0.012 Components of Primary Endpointb Death 8 (1.3) 6 (1.0) MI in surviving patients 49 (7.7) 24 (3.8) Urgent intervention in surviving patients without an acute MI 44 (6.9) 41 (6.6) The 30-day results are consistent with the results of the other three trials, with the greatest effects on the myocardial infarction and urgent intervention components of the composite endpoint. As secondary endpoints, the components of the composite endpoint were analyzed separately for the period prior to the PCI and the period from the beginning of the intervention through Day 30. The greatest difference in MI occurred in the post-intervention period: the rates of MI were lower in the Abciximab group compared with placebo (Abciximab 3.6%, placebo 6.1%). There was also a reduction in MI occurring prior to the PCI (Abciximab 0.6%, placebo 2.0%). An Abciximab-associated reduction in the incidence of urgent intervention occurred in the post-intervention period. No effect on mortality was observed in either period. At six months of follow up, the composite endpoint of death, MI, or all repeat intervention (urgent or non-urgent) was not different between the Abciximab and placebo groups (Abciximab 31.0%, placebo 30.8%, p=0.77).
Mortality was uncommon in all four trials. Similar mortality rates were observed in all arms within each trial. Patient follow-up through one year of the EPISTENT trial suggested decreased mortality among patients treated with Abciximab and stent placement compared to patients treated with stent alone (8/794 vs. 19/809, p=0.037). Data from earlier studies with balloon angioplasty were not suggestive of the same benefit. In all four trials, the rates of acute MI were significantly lower in the groups treated with Abciximab. Most of the Abciximab treatment effect was seen in reduction in the rate of acute non-Q-wave MI. Urgent intervention rates were also lower in Abciximab-treated groups in these trials.
Anticoagulation:
EPILOG and EPISTENT: Weight-adjusted low dose heparin, weight-adjusted Abciximab, careful vascular access site management and discontinuation of heparin after the procedure with early femoral arterial sheath removal were used.
The initial heparin bolus was based upon the results of the baseline ACT, according to the following regimen:
- ACT < 150 seconds: administer 70 U/kg heparin
- ACT 150 - 199 seconds: administer 50 U/kg heparin
- ACT ≥ 200 seconds: administer no heparin
Additional 20 U/kg heparin boluses were given to achieve and maintain an ACT of ≥ 200 seconds during the procedure.
Discontinuation of heparin immediately after the procedure and removal of the arterial sheath within six hours were strongly recommended in the trials. If prolonged heparin therapy or delayed sheath removal was clinically indicated, heparin was adjusted to keep the APTT at a target of 60 to 85 seconds (EPILOG) or 55 to 75 seconds (EPISTENT).
CAPTURE trial: Anticoagulation was initiated prior to the administration of Abciximab. Anticoagulation was initiated with an intravenous heparin infusion to achieve a target APTT of 60 to 85 seconds. The heparin infusion was not uniformly weight adjusted in this trial. The heparin infusion was maintained during the Abciximab infusion and was adjusted to achieve an ACT of 300 seconds or an APTT of 70 seconds during the PCI. Following the intervention, heparin management was as outlined above for the EPILOG trial.
-
INDICATIONS AND USAGE:
Abciximab is indicated as an adjunct to percutaneous coronary intervention for the prevention of cardiac ischemic complications
- in patients undergoing percutaneous coronary intervention
- in patients with unstable angina not responding to conventional medical therapy when percutaneous coronary intervention is planned within 24 hours
Safety and efficacy of Abciximab use in patients not undergoing percutaneous coronary intervention have not been established.
Abciximab is intended for use with aspirin and heparin and has been studied only in that setting, as described in CLINICAL STUDIES.
-
CONTRAINDICATIONS:
Because Abciximab may increase the risk of bleeding, Abciximab is contraindicated in the following clinical situations:
- Active internal bleeding
- Recent (within six weeks) gastrointestinal (GI) or genitourinary (GU) bleeding of clinical significance
- History of cerebrovascular accident (CVA) within two years, or CVA with a significant residual neurological deficit
- Bleeding diathesis
- Administration of oral anticoagulants within seven days unless prothrombin time is ≤ 1.2 times control
- Thrombocytopenia (< 100,000 cells/μL)
- Recent (within six weeks) major surgery or trauma
- Intracranial neoplasm, arteriovenous malformation, or aneurysm
- Severe uncontrolled hypertension
- Presumed or documented history of vasculitis
- Use of intravenous dextran before PCI, or intent to use it during an intervention
Abciximab is also contraindicated in patients with known hypersensitivity to any component of this product or to murine proteins.
-
WARNINGS:
Bleeding Events
Abciximab has the potential to increase the risk of bleeding events, rarely including those with a fatal outcome, particularly in the presence of anticoagulation, e.g., from heparin, other anticoagulants, or thrombolytics (see ADVERSE REACTIONS: Bleeding).
The risk of major bleeds due to Abciximab therapy is increased in patients receiving thrombolytics and should be weighed against the anticipated benefits.
Should serious bleeding occur that is not controllable with pressure, the infusion of Abciximab and any concomitant heparin should be stopped.
Allergic Reactions (including anaphylaxis)
Allergic reactions, some of which were anaphylaxis (sometimes fatal), have been reported rarely in patients treated with ReoPro. Patients with allergic reactions should receive appropriate treatment. Treatment of anaphylaxis should include immediate discontinuation of ReoPro administration and initiation of resuscitative measures.
-
PRECAUTIONS:
Bleeding Precautions- To minimize the risk of bleeding with Abciximab, it is important to use a low-dose, weight-adjusted heparin regimen, a weight-adjusted Abciximab bolus and infusion, strict anticoagulation guidelines, careful vascular access site management, discontinuation of heparin after the procedure and early femoral arterial sheath removal.
Therapy with Abciximab requires careful attention to all potential bleeding sites including catheter insertion sites, arterial and venous puncture sites, cutdown sites, needle puncture sites, and gastrointestinal, genitourinary, pulmonary (alveolar), and retroperitoneal sites.
Arterial and venous punctures, intramuscular injections, and use of urinary catheters, nasotracheal intubation, nasogastric tubes and automatic blood pressure cuffs should be minimized. When obtaining intravenous access, non-compressible sites (e.g., subclavian or jugular veins) should be avoided. Saline or heparin locks should be considered for blood drawing. Vascular puncture sites should be documented and monitored. Gentle care should be provided when removing dressings.
Femoral artery access site: Arterial access site care is important to prevent bleeding. Care should be taken when attempting vascular access that only the anterior wall of the femoral artery is punctured, avoiding a Seldinger (through and through) technique for obtaining sheath access. Femoral vein sheath placement should be avoided unless needed. While the vascular sheath is in place, patients should be maintained on complete bed rest with the head of the bed ≤ 30° and the affected limb restrained in a straight position. Patients may be medicated for back/groin pain as necessary.
Discontinuation of heparin immediately upon completion of the procedure and removal of the arterial sheath within six hours is strongly recommended if APTT ≤ 50 sec or ACT ≤ 175 sec (see PRECAUTIONS: Laboratory Tests). In all circumstances, heparin should be discontinued at least two hours prior to arterial sheath removal.
Following sheath removal, pressure should be applied to the femoral artery for at least 30 minutes using either manual compression or a mechanical device for hemostasis. A pressure dressing should be applied following hemostasis. The patient should be maintained on bed rest for six to eight hours following sheath removal or discontinuation of Abciximab, or four hours following discontinuation of heparin, whichever is later. The pressure dressing should be removed prior to ambulation. The sheath insertion site and distal pulses of affected leg(s) should be frequently checked while the femoral artery sheath is in place and for six hours after femoral artery sheath removal. Any hematoma should be measured and monitored for enlargement.
The following conditions have been associated with an increased risk of bleeding and may be additive with the effect of Abciximab in the angioplasty setting: PCI within 12 hours of the onset of symptoms for acute myocardial infarction, prolonged PCI (lasting more than 70 minutes) and failed PCI.
Use of Thrombolytics, Anticoagulants and Other Antiplatelet Agents- In the EPIC, EPILOG, CAPTURE, and EPISTENT trials, Abciximab was used concomitantly with heparin and aspirin. For details of the anticoagulation algorithms used in these clinical trials, see CLINICAL STUDIES: Anticoagulation. Because Abciximab inhibits platelet aggregation, caution should be employed when it is used with other drugs that affect hemostasis, including thrombolytics, oral anticoagulants, non-steroidal anti-inflammatory drugs, dipyridamole, and ticlopidine.
In the EPIC trial, there was limited experience with the administration of Abciximab with low molecular weight dextran. Low molecular weight dextran was usually given for the deployment of a coronary stent, for which oral anticoagulants were also given. In the 11 patients who received low molecular weight dextran with Abciximab, five had major bleeding events and four had minor bleeding events. None of the five placebo patients treated with low molecular weight dextran had a major or minor bleeding event (see CONTRAINDICATIONS).
Because of observed synergistic effects on bleeding, Abciximab therapy should be used judiciously in patients who have received systemic thrombolytic therapy. The GUSTO V trial randomized patients with acute myocardial infarction to treatment with combined Abciximab and half-dose Reteplase, or full-dose Reteplase alone (15). In this trial, the incidence of moderate or severe nonintracranial bleeding was increased in those patients receiving Abciximab and half-dose Reteplase versus those receiving Reteplase alone (4.6% versus 2.3%, respectively).
Thrombocytopenia- Thrombocytopenia, including severe thrombocytopenia, has been observed with Abciximab administration (see ADVERSE REACTIONS: Thrombocytopenia). Platelet counts should be monitored prior to, during, and after treatment with Abciximab (see PRECAUTIONS: Laboratory Tests). Acute decreases in platelet count should be differentiated between true thrombocytopenia and pseudothrombocytopenia (see PRECAUTIONS: Laboratory Tests). If true thrombocytopenia is verified, Abciximab should be immediately discontinued and the condition appropriately monitored and treated.
In clinical trials, patients who developed thrombocytopenia were followed with daily platelet counts until their platelet count returned to normal. Heparin and aspirin were discontinued for platelet counts below 60,000 cells/μL and platelets were transfused for a platelet count below 50,000 cells/μL. Most cases of severe thrombocytopenia (< 50,000 cells/μL) occurred within the first 24 hours of Abciximab administration, but some cases have been reported up to 2 weeks after administration.
In a registry study of Abciximab readministration, a history of thrombocytopenia associated with prior use of Abciximab was predictive of an increased risk of recurrent thrombocytopenia (see ADVERSE REACTIONS: Thrombocytopenia). Readministration within 30 days was associated with an increased incidence and severity of thrombocytopenia, as was a positive human anti-chimeric antibody (HACA) test at baseline, compared to the rates seen in studies with first administration.
Restoration of Platelet Function- In the event of serious uncontrolled bleeding or the need for emergency surgery, Abciximab should be discontinued. If platelet function does not return to normal, it may be restored, at least in part, with platelet transfusions.
Laboratory Tests- Before infusion of Abciximab, prothrombin time, ACT, APTT, and platelet count should be measured to identify pre-existing hemostatic abnormalities.
Based on an integrated analysis of data from all studies, the following guidelines may be utilized to minimize the risk for bleeding:
When Abciximab is initiated 18 to 24 hours before PCI, the APTT should be maintained between 60 and 85 seconds during the Abciximab and heparin infusion period.
During PCI the ACT should be maintained between 200 and 300 seconds.
If anticoagulation is continued in these patients following PCI, the APTT should be maintained between 55 and 75 seconds.
The APTT or ACT should be checked prior to arterial sheath removal. The sheath should not be removed unless APTT ≤ 50 seconds or ACT ≤ 175 seconds.
Platelet counts should be monitored prior to treatment, two to four hours following the bolus dose of Abciximab and at 24 hours or prior to discharge, whichever is first. If a patient experiences an acute platelet decrease (e.g., a platelet decrease to less than 100,000 cells/μL and a decrease of at least 25% from pre-treatment value), additional platelet counts should be determined. Platelet monitoring should continue until platelet counts return to normal.
To exclude pseudothrombocytopenia, a laboratory artifact due to in vitro anticoagulant interaction, blood samples should be drawn in three separate tubes containing ethylenediaminetetraacetic acid (EDTA), citrate and heparin, respectively. A low platelet count in EDTA but not in heparin and/or citrate is supportive of a diagnosis of pseudothrombocytopenia.
Readministration- Administration of Abciximab may result in the formation of HACA that could potentially cause allergic or hypersensitivity reactions (including anaphylaxis), thrombocytopenia or diminished benefit upon readministration of Abciximab (see WARNINGS: Allergic Reactions; see ADVERSE REACTIONS: Immunogenicity).
Readministration of Abciximab to patients undergoing PCI was assessed in a registry that included 1342 treatments in 1286 patients. Most patients were receiving their second Abciximab exposure; 15% were receiving the third or subsequent exposure. The overall rate of HACA positivity prior to the readministration was 6% and increased to 27% post-readministration. There were no reports of serious allergic reactions or anaphylaxis (see WARNINGS: Allergic Reactions). Thrombocytopenia was observed at higher rates in the readministration study than in the Phase 3 studies of first-time administration (see PRECAUTIONS: Thrombocytopenia and ADVERSE REACTIONS: Thrombocytopenia), suggesting that readministration may be associated with an increased incidence and severity of thrombocytopenia.
Drug Interactions- Formal drug interaction studies with Abciximab have not been conducted. Abciximab has been administered to patients with ischemic heart disease treated concomitantly with a broad range of medications used in the treatment of angina, myocardial infarction and hypertension. These medications have included heparin, warfarin, beta-adrenergic receptor blockers, calcium channel antagonists, angiotensin converting enzyme inhibitors, intravenous and oral nitrates, ticlopidine, and aspirin. Heparin, other anticoagulants, thrombolytics, and antiplatelet agents are associated with an increase in bleeding. Patients with HACA titers may have allergic or hypersensitivity reactions when treated with other diagnostic or therapeutic monoclonal antibodies.
Carcinogenesis, Mutagenesis and Impairment of Fertility- In vitro and in vivo mutagenicity studies have not demonstrated any mutagenic effect. Long-term studies in animals have not been performed to evaluate the carcinogenic potential or effects on fertility in male or female animals.
Pregnancy- Animal reproduction studies have not been conducted with Abciximab. It is also not known whether Abciximab can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Abciximab should be given to a pregnant woman only if clearly needed.
Nursing Mothers- It is not known whether this drug is excreted in human milk or absorbed systemically after ingestion. Because many drugs are excreted in human milk, caution should be exercised when Abciximab is administered to a nursing woman.
Geriatric Use- Of the total number of 7860 patients in the four Phase 3 trials, 2933 (37%) were 65 and over, while 653 (8%) were 75 and over. No overall differences in safety or efficacy were observed between patients of age 65 to less than 75 as compared to younger patients. The clinical experience is not adequate to determine whether patients of age 75 or greater respond differently than younger patients.
-
ADVERSE REACTIONS:
Bleeding- Abciximab has the potential to increase the risk of bleeding, particularly in the presence of anticoagulation, e.g., from heparin, other anticoagulants or thrombolytics. Bleeding in the Phase 3 trials was classified as major, minor or insignificant by the criteria of the Thrombolysis in Myocardial Infarction study group (16). Major bleeding events were defined as either an intracranial hemorrhage or a decrease in hemoglobin greater than 5 g/dL. Minor bleeding events included spontaneous gross hematuria, spontaneous hematemesis, observed blood loss with a hemoglobin decrease of more than 3 g/dL, or a decrease in hemoglobin of at least 4 g/dL without an identified bleeding site. Insignificant bleeding events were defined as a decrease in hemoglobin of less than 3 g/dL or a decrease in hemoglobin between 3-4 g/dL without observed bleeding. In patients who received transfusions, the number of units of blood lost was estimated through an adaptation of the method of Landefeld, et al. (17).
In the EPIC trial, in which a non-weight-adjusted, longer-duration heparin dose regimen was used, the most common complication during Abciximab therapy was bleeding during the first 36 hours. The incidences of major bleeding, minor bleeding and transfusion of blood products were significantly increased. Major bleeding occurred in 10.6% of patients in the Abciximab bolus plus infusion arm compared with 3.3% of patients in the placebo arm. Minor bleeding was seen in 16.8% of Abciximab bolus plus infusion patients and 9.2% of placebo patients (7). Approximately 70% of Abciximab-treated patients with major bleeding had bleeding at the arterial access site in the groin. Abciximab-treated patients also had a higher incidence of major bleeding events from gastrointestinal, genitourinary, retroperitoneal, and other sites.
Bleeding rates were reduced in the CAPTURE trial, and further reduced in the EPILOG and EPISTENT trials by use of modified dosing regimens and specific patient management techniques. In EPILOG and EPISTENT, using the heparin and Abciximab dosing, sheath removal and arterial access site guidelines described under PRECAUTIONS, the incidence of major bleeding in patients treated with Abciximab and low-dose, weight-adjusted heparin was not significantly different from that in patients receiving placebo.
Subgroup analyses in the EPIC and CAPTURE trials showed that non-CABG major bleeding was more common in Abciximab patients weighing ≤ 75 kg. In the EPILOG and EPISTENT trials, which used weight-adjusted heparin dosing, the non-CABG major bleeding rates for Abciximab-treated patients did not differ substantially by weight subgroup.
Although data are limited, Abciximab treatment was not associated with excess major bleeding in patients who underwent CABG surgery. (The range among all treatment arms was 3-5% in EPIC, and 1-2% in the CAPTURE, EPILOG, and EPISTENT trials.) Some patients with prolonged bleeding times received platelet transfusions to correct the bleeding time prior to surgery. (see PRECAUTIONS: Restoration of Platelet Function.)
The rates of major bleeding, minor bleeding and bleeding events requiring transfusions in the CAPTURE, EPILOG, and EPISTENT trials are shown in Table 4. The rates of insignificant bleeding events are not included in Table 4.
Cases of fatal bleeding have been reported rarely during post-marketing use of Abciximab (see WARNINGS: Bleeding Events).
Pulmonary alveolar hemorrhage has been rarely reported during use of Abciximab. This can present with any or all of the following in close association with ReoPro administration: hypoxemia, alveolar infiltrates on chest x-ray, hemoptysis, or an unexplained drop in hemoglobin.
Table 4 NON-CABG BLEEDING IN TRIALS OF PERCUTANEOUS CORONARY INTERVENTION (EPILOG, EPISTENT and CAPTURE) Number of Patients with Bleeds (%) a Patients who had bleeding in more than one classification are counted only once according to the most severe classification. Patients with multiple bleeding events of the same classification are also counted once within that classification.
b Patients with major non-CABG bleeding who received packed red blood cells or whole blood transfusion.
c Standard-dose heparin with or without stent (EPILOG and EPISTENT)
d Low-dose heparin with or without stent (EPILOG and EPISTENT)
e Standard-dose heparin (EPILOG)
f Standard-dose heparin (CAPTURE)
EPILOG and EPISTENT:
Placeboc
(n=1748)Abciximab +
Low-dose Heparind
(n=2525)Abciximab +
Standard-dose Heparine
(n=918)Majora 18 (1.0) 21 (0.8) 17 (1.9) Minor 46 (2.6) 82 (3.2) 70 (7.6) Requiring transfusionb 15 (0.9) 13 (0.5) 7 (0.8)
CAPTURE:Placebof
(n=635)Abciximabf
(n=630)Majora 12 (1.9) 24 (3.8) Minor 13 (2.0) 30 (4.8) Requiring transfusionb 9 (1.4) 15 (2.4) Intracranial Hemorrhage and Stroke- The total incidence of intracranial hemorrhage and non-hemorrhagic stroke across all four trials was not significantly different, 9/3023 for placebo patients and 15/4680 for Abciximab-treated patients. The incidence of intracranial hemorrhage was 3/3023 for placebo patients and 7/4680 for Abciximab patients.
Thrombocytopenia- In the clinical trials, patients treated with Abciximab were more likely than patients treated with placebo to experience decreases in platelet counts.
Among patients in the EPILOG and EPISTENT trials who were treated with Abciximab plus low-dose heparin, the proportion of patients with any thrombocytopenia (platelets less than 100,000 cells/μL) ranged from 2.5 to 3.0%. The incidence of severe thrombocytopenia (platelets less than 50,000 cells/μL) ranged from 0.4 to 1.0% and platelet transfusions were required in 0.9 to 1.1%, respectively. Modestly lower rates were observed among patients treated with placebo plus standard-dose heparin. Overall higher rates were observed among patients in the EPIC and CAPTURE trials treated with Abciximab plus longer duration heparin: 2.6 to 5.2% were found to have any thrombocytopenia, 0.9 to 1.7% had severe thrombocytopenia, and 2.1 to 5.5% required platelet transfusion, respectively.
In a readministration registry study of patients receiving a second or subsequent exposure to Abciximab (see PRECAUTIONS: Readministration) the incidence of any degree of thrombocytopenia was 5%, with an incidence of profound thrombocytopenia of 2% (<20,000 cell/μL). Factors associated with an increased risk of thrombocytopenia were a history of thrombocytopenia on previous Abciximab exposure, readministration within 30 days, and a positive HACA assay prior to the readministration.
Among 14 patients who had thrombocytopenia associated with a prior exposure to Abciximab, 7 (50%) had recurrent thrombocytopenia. In 130 patients with a readministration interval of 30 days or less, 25 (19%) developed thrombocytopenia. Severe thrombocytopenia occurred in 19 of these patients. Among the 71 patients who had a positive HACA assay at baseline, 11 (15%) developed thrombocytopenia, 7 of which were severe.
Allergic Reactions- There have been rare reports of allergic reactions, some of which were anaphylaxis (see WARNINGS: Allergic Reactions).
Other Adverse Reactions- Table 5 shows adverse events other than bleeding and thrombocytopenia from the combined EPIC, EPILOG and CAPTURE trials which occurred in patients in the bolus plus infusion arm at an incidence of more than 0.5% higher than in those treated with placebo.
Table 5 ADVERSE EVENTS AMONG TREATED PATIENTS IN THE EPIC, EPILOG, AND CAPTURE TRIALS
EventPlacebo
(n=2226)Bolus + Infusion
(n=3111)Number of Patients (%) Cardiovascular system Hypotension 230 (10.3) 447 (14.4) Bradycardia 79 (3.5) 140 (4.5) Gastrointestinal system Nausea 255 (11.5) 423 (13.6) Vomiting 152 (6.8) 226 (7.3) Abdominal pain 49 (2.2) 97 (3.1) Miscellaneous Back pain 304 (13.7) 546 (17.6) Chest pain 208 (9.3) 356 (11.4) Headache 122 (5.5) 200 (6.4) Puncture site pain 58 (2.6) 113 (3.6) Peripheral edema 25 (1.1) 49 (1.6) The following additional adverse events from the EPIC, EPILOG and CAPTURE trials were reported by investigators for patients treated with a bolus plus infusion of Abciximab at incidences which were less than 0.5% higher than for patients in the placebo arm.
Cardiovascular System: ventricular tachycardia (1.4%), pseudoaneurysm (0.8%), palpitation (0.5%), arteriovenous fistula (0.4%), incomplete AV block (0.3%), nodal arrhythmia (0.2%), complete AV block (0.1%), embolism (limb) (0.1%), thrombophlebitis (0.1%);
Gastrointestinal System: dyspepsia (2.1%), diarrhea (1.1%), ileus (0.1%), gastroesophogeal reflux (0.1%);
Hemic and Lymphatic System: anemia (1.3%), leukocytosis (0.5%), petechiae (0.2%);
Nervous System: dizziness (2.9%), anxiety (1.7%), abnormal thinking (1.3%), agitation (0.7%), hypesthesia (0.6%), confusion (0.5%), muscle contractions (0.4%), coma (0.2%), hypertonia (0.2%), diplopia (0.1%);
Respiratory System: pneumonia (0.4%), rales (0.4%), pleural effusion (0.3%), bronchitis (0.3%), bronchospasm (0.3%), pleurisy (0.2%), pulmonary embolism (0.2%), rhonchi (0.1%);
Musculoskeletal System: myalgia (0.2%);
Urogenital System: urinary retention (0.7%), dysuria (0.4%), abnormal renal function (0.4%), frequent micturition (0.1%), cystalgia (0.1%), urinary incontinence (0.1%), prostatitis (0.1%);
Miscellaneous: pain (5.4%), sweating increased (1.0%), asthenia (0.7%), incisional pain (0.6%), pruritus (0.5%), abnormal vision (0.3%), edema (0.3%), wound (0.2%), abscess (0.2%), cellulitis (0.2%), peripheral coldness (0.2%), injection site pain (0.1%), dry mouth (0.1%), pallor (0.1%), diabetes mellitus (0.1%), hyperkalemia (0.1%), enlarged abdomen (0.1%), bullous eruption (0.1%), inflammation (0.1%), drug toxicity (0.1%).
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. In the EPIC, EPILOG, and CAPTURE trials, positive HACA responses occurred in approximately 5.8% of these patients receiving a first exposure to Abciximab. No increase in hypersensitivity or allergic reactions was observed with Abciximab treatment (see WARNINGS: Allergic Reactions).
In a study of readministration of Abciximab to patients (see PRECAUTIONS: Readministration) the overall rate of HACA positivity prior to the readministration was 6% and increased post-readministration to 27%. Among the 36 subjects receiving a fourth or greater Abciximab exposure, HACA positive assays were observed post-readministration in 16 subjects (44%). There were no reports of serious allergic reactions or anaphylaxis (see WARNINGS: Allergic Reactions). HACA positive status was associated with an increased risk of thrombocytopenia (see PRECAUTIONS: Thrombocytopenia).
The data reflect the percentage of patients whose test results were considered positive for antibodies to Abciximab using an ELISA assay, and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Abciximab with the incidence of antibodies to other products may be misleading.
- OVERDOSAGE:
-
DOSAGE AND ADMINISTRATION:
The safety and efficacy of Abciximab have only been investigated with concomitant administration of heparin and aspirin as described in CLINICAL STUDIES.
In patients with failed PCIs, the continuous infusion of Abciximab should be stopped because there is no evidence for Abciximab efficacy in that setting.
In the event of serious bleeding that cannot be controlled by compression, Abciximab and heparin should be discontinued immediately.
The recommended dosage of Abciximab in adults is a 0.25 mg/kg intravenous bolus administered 10-60 minutes before the start of PCI, followed by a continuous intravenous infusion of 0.125 μg/kg/min (to a maximum of 10 μg/min) for 12 hours.
Patients with unstable angina not responding to conventional medical therapy and who are planned to undergo PCI within 24 hours may be treated with an Abciximab 0.25 mg/kg intravenous bolus followed by an 18- to 24-hour intravenous infusion of 10 μg/min, concluding one hour after the PCI.
Instructions for Administration
- Parenteral drug products should be inspected visually for particulate matter prior to administration. Preparations of Abciximab containing visibly opaque particles should NOT be used.
- Hypersensitivity reactions should be anticipated whenever protein solutions such as Abciximab are administered. Epinephrine, dopamine, theophylline, antihistamines and corticosteroids should be available for immediate use. If symptoms of an allergic reaction or anaphylaxis appear, the infusion should be stopped and appropriate treatment given (see WARNINGS: Allergic Reactions).
- As with all parenteral drug products, aseptic procedures should be used during the administration of Abciximab.
- Withdraw the necessary amount of Abciximab for bolus injection into a syringe. Filter the bolus injection using a sterile, non-pyrogenic, low protein-binding 0.2 or 5 μm syringe filter. Discard any unused portion left in the vial.
- Withdraw the necessary amount of Abciximab for the continuous infusion into a syringe. Inject into an appropriate container of sterile 0.9% saline or 5% dextrose and infuse at the calculated rate via a continuous infusion pump. The continuous infusion should be filtered either upon admixture using a sterile, non-pyrogenic, low protein-binding 0.2 or 5 μm syringe filter or upon administration using an in-line, sterile, non-pyrogenic, low protein-binding 0.2 or 0.22 μm filter. Discard the unused portion at the end of the infusion.
- No incompatibilities have been shown with intravenous infusion fluids or commonly used cardiovascular drugs. Nevertheless, Abciximab should be administered in a separate intravenous line whenever possible and not mixed with other medications.
- No incompatibilities have been observed with glass bottles or polyvinyl chloride bags and administration sets.
- HOW SUPPLIED:
-
REFERENCES:
- 1. Reverter JC, Beguin S, Kessels H, Kumar R, Hemmer HC, Coller BS. Inhibition of platelet-mediated, tissue-factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody; potential implications for the effect of c7E3 Fab treatment on acute thrombosis and "clinical restenosis". J Clin Invest. 1996;98:863-874.
- 2. Alteri D, Edgington T, A monoclonal antibody reacting with distinct adhesion molecules defines a transition in the functional state of the receptor CD11b/CD18 (Mac-1). J Immunol. 1988;141:2656-2660.
- 3. Simon DI, Xu H, Ortlepp S, Rogers C, Rao NK. 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1. Arterioscler Thromb Vasc Biol. 1997;17:528-535.
- 4. Mickelson JK, Ali MN, Kleiman NS, Lakkis NM, Chow TW, Hughes BJ. Chimeric 7E3 Fab (ReoPro) decreases detectable CD11b on neutrophils from patients undergoing coronary angioplasty. J Am Coll Cardiol. 1999;33:97-106.
- 5. Tcheng J, Ellis SG, George BS. Pharmacodynamics of chimeric glycoprotein IIb/IIIa integrin antiplatelet antibody Fab 7E3 in high risk coronary angioplasty. Circulation. 1994;90:1757- 1764.
- 6. Simoons ML, de Boer MJ, van der Brand MJBM, et al. Randomized trial of a GPIIb/IIIa platelet receptor blocker in refractory unstable angina. Circulation. 1994;89:596-603.
- 7. EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994;330:956-961.
- 8. Topol EJ, Califf RM, Weisman HF, et al. Randomised trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. Lancet. 1994;343:881-886.
- 9. Topol EJ, Ferguson JJ, Weisman HF, et al. for the EPIC Investigators. Long-term protection from myocardial ischemic events in a randomized trial of brief integrin blockade with percutaneous coronary intervention. JAMA. 1997;278:479-484.
- 10. EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low dose heparin during percutaneous coronary revascularization. N Engl J Med. 1997;336:1689-1696.
- 11. Lincoff AM, Tcheng JE, Califf RM, et al. for the EPILOG Investigators. Sustained suppression of ischemic complications of coronary intervention by platelet GP IIb/IIIa blockade with abciximab. Circ. 1999;99:1951-1958.
- 12. EPISTENT Investigators. Randomised placebo-controlled and balloon angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet. 1998;352:87-92.
- 13. Lincoff AM, Califf RM, Moliterno DJ, et al. for the EPISTENT Investigator. Complementary clinical benefits of coronary stenting and blockade of platelet glycoprotein IIb/IIIa receptors. N Engl J Med. 1999;341:319-327.
- 14. CAPTURE Investigators. Randomised placebo-controlled trial of abciximab before, during and after coronary intervention in refractory unstable angina: the CAPTURE study. Lancet. 1997;349:1429-1435.
- 15. Data on file.
- 16. Rao, AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial - Phase I: Hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11:1-11.
- 17. Landefeld, CS, Cook EF, Flatley M, et al. Identification and preliminary validation of predictors of major bleeding in hospitalized patients starting anticoagulant therapy. Am J Med. 1987;82:703-713.
Product of The Netherlands
Manufactured by:
Janssen Biotech, Inc.
Horsham, PA 19044
U.S. License Number: 1864© 2016 Janssen Pharmaceutical Companies
Revision Date: August 2019
-
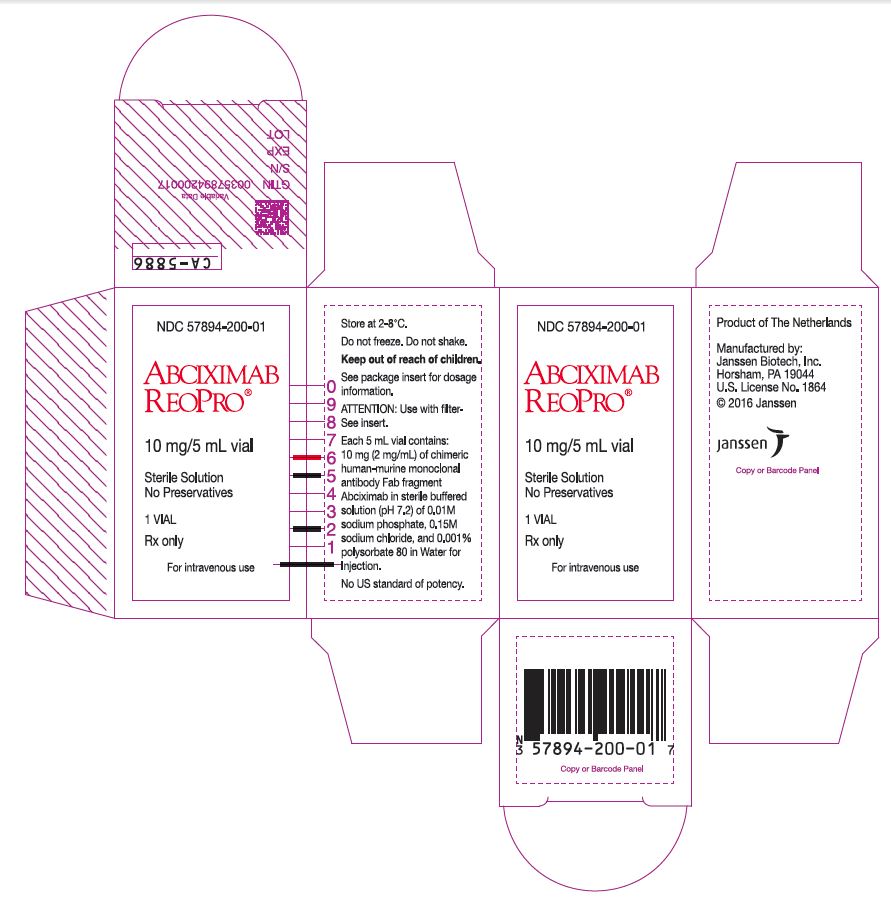
PRINCIPAL DISPLAY PANEL - 10 mg/5 mL Vial Box
NDC: 57894-200-01
ABCIXIMAB
REOPRO®10 mg/5 mL vial
Sterile Solution
No Preservatives1 VIAL
Rx only
For intravenous use
-
INGREDIENTS AND APPEARANCE
REOPRO
abciximab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57894-200 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength abciximab (UNII: X85G7936GV) (abciximab - UNII:X85G7936GV) abciximab 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium phosphate (UNII: SE337SVY37) sodium chloride (UNII: 451W47IQ8X) polysorbate 80 (UNII: 6OZP39ZG8H) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57894-200-01 1 in 1 BOX 01/03/2017 1 5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103575 01/03/2017 Labeler - Janssen Biotech, Inc. (099091753) Establishment Name Address ID/FEI Business Operations Janssen Biologics B.V. 409612918 API MANUFACTURE(57894-200) Establishment Name Address ID/FEI Business Operations Hospira Inc. 030606222 MANUFACTURE(57894-200)
Trademark Results [REOPRO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REOPRO 74512074 2022377 Live/Registered |
JANSSEN BIOTECH, INC. 1994-04-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.