Encare by Blairex Laboratories, Inc.
Encare by
Drug Labeling and Warnings
Encare by is a Otc medication manufactured, distributed, or labeled by Blairex Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ENCARE- nonoxynol-9 insert
Blairex Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
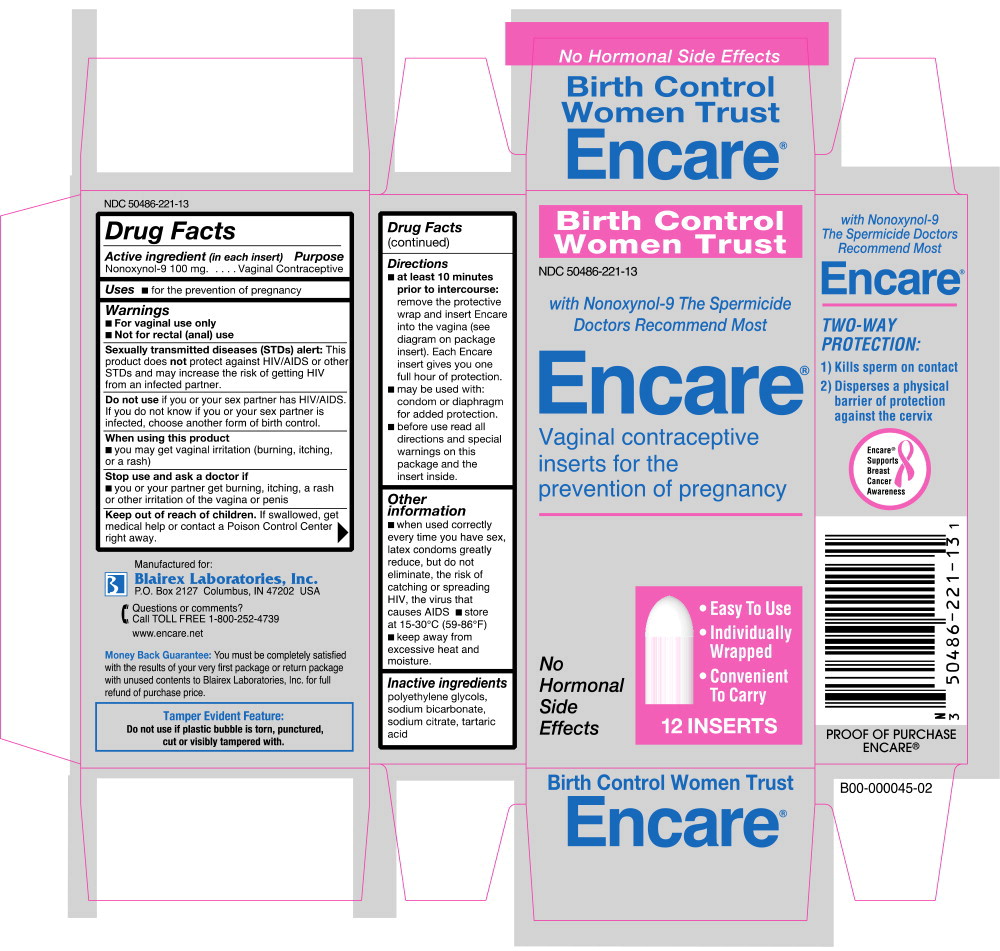
Warnings
- For vaginal use only
- Not for rectal (anal) use
Sexually transmitted diseases (STDs) alert: This product does not protect against HIV/AIDS or other STDs and may increase the risk of getting HIV from an infected partner.
Do not use if you or your sex partner has HIV/AIDS. If you do not know if you or your sex partner is infected, choose another form of birth control.
Directions
- at least 10 minutes prior to intercourse: remove the protective wrap and insert Encare into the vagina (see diagram on package insert). Each Encare insert gives you one full hour of protection.
- may be used with: condom or diaphragm for added protection.
- before use: read all directions and special warnings on this package and the insert inside.
Other information
- when used correctly every time you have sex, latex condoms greatly reduce, but do not eliminate, the risk of catching or spreading HIV, the virus that causes AIDS
- store at 15-30°C (59-86°F)
- keep away from excessive heat and moisture.
Inactive ingredients
polyethylene glycols, sodium bicarbonate, sodium citrate, tartaric acid.
Manufactured for: Blairex Laboratories, Inc. P.O. Box 2127 Columbus, IN 47202 USA
Principal Display Panel - Carton Label
NDC: 50486-221-18
with Nonoxynol-9
The Spermicide Doctors Recommend Most
Encare®
Vaginal contraceptive inserts
for prevention of pregnancy
No Hormonal Side Effects
- Easy To Use
- Individually Wrapped
- Convenient To Carry
18 INSERTS
Principal Display Panel - Carton Label
NDC: 50486-221-12
with Nonoxynol-9
The Spermicide Doctors Recommend Most
Encare®
Vaginal contraceptive inserts
for prevention of pregnancy
No Hormonal Side Effects
- Easy To Use
- Individually Wrapped
- Convenient To Carry
12 INSERTS
Principal Display Panel - Carton Label
Birth Control
Women Trust
NDC: 50486-221-13
with Nonoxynol-9 The Spermicide
Doctors Recommend Most
Encare®
Vaginal contraceptive
inserts for the
prevention of pregnancy
No
Hormonal
Side
Effects
- Easy To Use
- Individually Wrapped
- Convenient To Carry
12 INSERTS
| ENCARE
nonoxynol-9 insert |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Blairex Laboratories, Inc. (092575133) |
 Questions or comments? Call TOLL FREE 1-800-252-4739
Questions or comments? Call TOLL FREE 1-800-252-4739