Pralatrexate by Fresenius Kabi USA, LLC / Acrotech Biopharma Inc. / Baxter Oncology, GmbH, (Baxter) PRALATREXATE injection
Pralatrexate by
Drug Labeling and Warnings
Pralatrexate by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC, Acrotech Biopharma Inc., Baxter Oncology, GmbH, (Baxter). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PRALATREXATE INJECTION safely and effectively. See full prescribing information for PRALATREXATE INJECTION.
PRALATREXATE injection, for intravenous use
Initial U.S. Approval: 2009INDICATIONS AND USAGE
Pralatrexate injection is a dihydrofolate reductase inhibitor indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL).
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
DOSAGE AND ADMINISTRATION
- Supplement patients with vitamin B12 mg intramuscularly every 8-10 weeks starting 10 weeks before the first dose and folic acid 1 to 1.25 mg orally once daily starting 10 days before the first dose. (2.1)
- The recommended dosage of Pralatrexate injection is 30 mg/m2 intravenously over 3 to 5 minutes once weekly for 6 weeks in 7-week cycles. (2.1)
- For patients with severe renal impairment (GFR 15 to 29 mL/min/1.73 m2), reduce the Pralatrexate injection dose to 15 mg/m2 (2.1).
DOSAGE FORMS AND STRENGTHS
Injection: 20 mg/1 mL or 40 mg/2 mL in a single-dose vial (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Myelosuppression: Monitor complete blood counts and omit and/or reduce dose based on ANC and platelet count. (2.4, 5.1)
- Mucositis:Monitor at least weekly. Omit and/or reduce dose for grade 2 or higher mucositis. (2.4, 5.2)
- Dermatologic reactions: Reactions, including fatal reactions, occurred and may be progressive and increase in severity with further treatment. Monitor closely and withhold or discontinue Pralatrexate injection based on severity. (2.4, 5.3)
- Tumor lysis syndrome: Monitor patients who are increased risk and treat promptly. (5.4)
- Hepatic toxicity: Monitor for liver function tests. Omit until recovery, adjust or discontinue therapy based on severity. (2.4, 5.5)
- Risk of increased toxicity with renal impairment: Avoid Pralatrexate injection in patients with end stage renal disease with or without dialysis. If the potential benefit of administration justifies the potential risk, monitor renal function and reduce the Pralatrexate injection dose based on adverse reactions. (2.3, 2.4, 5.6)
- Embryo-fetal toxicity: Can cause fetal harm. Advise patients of the potential risk to a fetus and to use an effective method of contraception. (5.7, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (>35%) are mucositis, thrombocytopenia, nausea, and fatigue. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Avoid coadministration with probenecid or nonsteroidal anti-inflammatory drugs. If coadministration is unavoidable, monitor for increased risk of adverse reactions. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
2.2 Recommended Dosage
2.3 Dosage Modifications for Renal Impairment and End Stage Renal Disease
2.4 Monitoring and Dosage Modifications for Adverse Reactions
2.5 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Mucositis
5.3 Dermatologic Reactions
5.4 Tumor Lysis Syndrome
5.5 Hepatic Toxicity
5.6 Risk of Increased Toxicity with Renal Impairment
5.7 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Pralatrexate Injection
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Pralatrexate injection is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL).
This indication is approved under accelerated approval based on overall response rate [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
Pretreatment Vitamin Supplementation
2.2 Recommended Dosage
The recommended dosage of Pralatrexate injection is 30 mg/m2 intravenously over 3-5 minutes once weekly for 6 weeks in 7-week cycles until progressive disease or unacceptable toxicity.
2.3 Dosage Modifications for Renal Impairment and End Stage Renal Disease
- Severe renal impairment (eGFR 15 to 29 mL/min/1.73 m2 by MDRD): Reduce the Pralatrexate injection dose to 15 mg/m2 [see Use in Specific Populations (8.6)].
- End stage renal disease (ESRD: eGFR less than 15 mL/min/1.73 m2 by MDRD) with or without dialysis: Avoid administration. If the potential benefit of administration justifies the potential risk, monitor renal function and reduce the Pralatrexate injection dose based on adverse reactions [see Warnings and Precautions (5.6), Use in Specific Populations (8.6)].
2.4 Monitoring and Dosage Modifications for Adverse Reactions
Monitoring
Monitor complete blood cell counts and severity of mucositis at baseline and weekly. Perform serum chemistry tests, including renal and hepatic function, prior to the start of the first and fourth dose of each cycle.
Recommended Dosage Modifications
Do not administer Pralatrexate injection until:
- Mucositis Grade 1 or less.
- Platelet of 100,000/mcL or greater for first dose and 50,000/mcL or greater for all subsequent doses.
- Absolute neutrophil count (ANC) of 1,000/mcL or greater.
Dosage modifications for adverse reactions are provided in Tables 1, 2, and 3.
Table 1 Pralatrexate Injection Dosage Modifications for Mucositis a Based National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 3.0)
Mucositis Gradea on Day of Treatment Action Recommended Dose upon Recovery to Grade 0 or 1 Patients Without Severe Renal Impairment Patients with Severe Renal Impairment Grade 2 Omit dose Continue prior dose Continue prior dose Grade 2 recurrence Omit dose 20 mg/m2 10 mg/m2 Grade 3 Omit dose 20 mg/m2 10 mg/m2 Grade 4 Stop therapy Table 2 Pralatrexate Injection Dosage Modifications for Myelosuppression G-CSF=granulocyte colony-stimulating factor; GM-CSF=granulocyte macrophage colony-stimulating factor
Blood Count on Day of Treatment Duration of Toxicity Action Recommended Dose Upon Recovery Patients Without Severe Renal Impairment Patients with Severe Renal Impairment Platelet less than 50,000/mcL 1 week Omit dose Continue prior dose Continue prior dose 2 weeks Omit dose 20 mg/m2 10 mg/m2 3 weeks Stop therapy ANC 500 to 1,000/mcL and no fever 1 week Omit dose Continue prior dose Continue prior dose ANC 500 to 1,000/mcL with fever or ANC less than 500/mcL 1 week Omit dose, give G-CSF or GM-CSF Continue prior dose with G-CSF or GM-CSF Continue prior dose with G-CSF or GM-CSF 2 weeks or recurrence Omit dose, give G-CSF or GM-CSF 20 mg/m2 with G-CSF or GM-CSF 10 mg/m2 with G-CSF or GM-CSF 3 weeks or
2nd recurrenceStop therapy Table 3 Pralatrexate Injection Dosage Modifications for All Other Adverse Reactions a Based on NCI CTCAE version 3.0
Toxicity Gradea on Day of Treatment Action Recommended Dose upon Recovery to Grade 2 or Lower Patients Without Severe Renal Impairment Patients with Severe Renal Impairment Grade 3 Omit dose 20 mg/m2 10 mg/m2 Grade 4 Stop therapy 2.5 Preparation and Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use any vials exhibiting particulate matter or discoloration.
Pralatrexate injection is a hazardous drug. Follow applicable special handling and disposal procedures.1 If Pralatrexate injection comes in contact with the skin, immediately and thoroughly wash with soap and water. If Pralatrexate injection comes in contact with mucous membranes, flush thoroughly with water.
Aseptically withdraw the calculated dose from the appropriate number of vial(s) into a syringe for immediate use. Do not dilute Pralatrexate injection.
Administer undiluted Pralatrexate injection intravenously over 3-5 minutes via the side port of a free-flowing 0.9% Sodium Chloride Injection.
After withdrawal of dose, discard vial(s) including any unused portion.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Pralatrexate injection can cause myelosuppression, manifested by thrombocytopenia, neutropenia, and/or anemia.
Administer vitamin B12 and instruct patients to take folic acid to reduce the risk of treatment-related myelosuppression [see Dosage and Administration (2.1)].
Monitor complete blood counts and omit and/or reduce the dose based on ANC and platelet count prior to each dose [see Dosage and Administration (2.4)].
5.2 Mucositis
Pralatrexate injection can cause mucositis [see Adverse Reactions (6.1)].
Administer vitamin B12 and instruct patients to take folic acid to reduce the risk of mucositis [see Dosage and Administration (2.1)].
Monitor for mucositis weekly and omit and/or reduce the dose for grade 2 or higher mucositis [see Dosage and Administration (2.4)].
5.3 Dermatologic Reactions
Pralatrexate injection can cause severe dermatologic reactions, which may result in death. These dermatologic reactions have been reported in clinical studies (2.1% of 663 patients) and post marketing experience, and have included skin exfoliation, ulceration, and toxic epidermal necrolysis (TEN) [see Adverse Reactions (6.1, 6.2)]. They may be progressive and increase in severity with further treatment and may involve skin and subcutaneous sites of known lymphoma.
Monitor closely for dermatologic reactions. Withhold or discontinue Pralatrexate injection based on severity [see Dosage and Administration (2.4)].
5.4 Tumor Lysis Syndrome
Pralatrexate injection can cause tumor lysis syndrome (TLS). Monitor patients who are at increased risk of TLS and treat promptly.
5.5 Hepatic Toxicity
Pralatrexate injection can cause hepatic toxicity and liver function test abnormalities [see Adverse Reactions (6.1)]. Persistent liver function test abnormalities may be indicators of hepatic toxicity and require dose modification or discontinuation.
Monitor liver function tests. Omit dose until recovery, adjust or discontinue therapy based on the severity of the hepatic toxicity [see Dosage and Administration (2.4)].
5.6 Risk of Increased Toxicity with Renal Impairment
Patients with severe renal impairment (eGFR 15 to < 30 mL/min/1.73 m2 based on MDRD) may be at greater risk for increased exposure and adverse reactions. Reduce Pralatrexate injection dosage in patients with severe renal impairment [see Dosage and Administration (2.3)].
Serious adverse reactions, including TEN and mucositis, were reported in patients with end stage renal disease (ESRD) undergoing dialysis who were administered Pralatrexate injection. Avoid Pralatrexate injection in patients with ESRD with or without dialysis. If the potential benefit of administration justifies the potential risk, monitor renal function and reduce the Pralatrexate injection dose based on adverse reactions [see Dosage and Administration (2.3)].
5.7 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, Pralatrexate injection can cause fetal harm when administered to a pregnant woman. Pralatrexate injection was embryotoxic and fetotoxic in rats and rabbits. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Pralatrexate injection and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Pralatrexate injection and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Mucositis [see Warnings and Precautions (5.2)]
- Dermatologic Reactions [see Warnings and Precautions (5.3)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.4)]
- Hepatic Toxicity [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Peripheral T-cell Lymphoma
The safety of Pralatrexate injection was evaluated in Study PDX-008 [see Clinical Studies (14)]. Patients received Pralatrexate injection 30 mg/m2 once weekly for 6 weeks in 7-week cycles. The median duration of treatment was 70 days (range: 1 day to 1.5 years). The majority of patients (69%, n = 77) remained at the target dose for the duration of treatment. Overall, 85% of scheduled doses were administered.
Forty-four percent of patients (n = 49) experienced a serious adverse event while on study or within 30 days after their last dose of Pralatrexate injection. The most common serious adverse events (> 3%), regardless of causality, were pyrexia, mucositis, sepsis, febrile neutropenia, dehydration, dyspnea, and thrombocytopenia. One death from cardiopulmonary arrest in a patient with mucositis and febrile neutropenia was reported in this trial. Across clinical trials, deaths from mucositis, febrile neutropenia, sepsis, and pancytopenia occurred in 1.2% of patients who received doses ranging from 30 mg/m2 to 325 mg/m2.
Twenty-three percent of patients (n = 25) discontinued treatment with Pralatrexate injection due to adverse reactions. The most frequent adverse reactions reported as the reason for discontinuation of treatment were mucositis (6%) and thrombocytopenia (5%).
The most common adverse reactions (> 35%) were mucositis, thrombocytopenia, nausea, and fatigue.
Table 4 summarizes the adverse reactions in Study PDX-008.
Table 4 Adverse Reactions in (≥ 10%) in Patients Who Received Pralatrexate Injection in Study PDX-008 a Mucositis includes stomatitis or mucosal inflammation of the gastrointestinal and genitourinary tracts.
b Five patients with platelets < 10,000/mcL.
c Liver function test abnormal includes increased ALT, increased AST, and increased transaminases
Pralatrexate Injection
N=111All Grades (%) Grade 3 (%) Grade 4 (%) Any Adverse Reaction 100 43 31 Mucositisa 70 17 4 Thrombocytopeniab 41 14 19b Nausea 40 4 0 Fatigue 36 5 2 Anemia 34 15 2 Constipation 33 0 0 Pyrexia 32 1 1 Edema 30 1 0 Cough 28 1 0 Epistaxis 26 0 0 Vomiting 25 2 0 Neutropenia 24 13 7 Diarrhea 21 2 0 Dyspnea 19 7 0 Anorexia 15 3 0 Hypokalemia 15 4 1 Rash 15 0 0 Pruritus 14 2 0 Pharyngolaryngeal pain 14 1 0 Liver function test
abnormalc13 5 0 Abdominal pain 12 4 0 Pain in extremity 12 0 0 Back pain 11 3 0 Leukopenia 11 3 4 Night sweats 11 0 0 Asthenia 10 1 0 Upper respiratory tract
infection10 1 0 Tachycardia 10 0 0 6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Pralatrexate injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic Reactions: Toxic epidermal necrolysis.
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Pralatrexate Injection
Coadministration of Pralatrexate injection with probenecid increased pralatrexate plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions. Avoid coadministration with probenecid or nonsteroidal anti-inflammatory drugs. If coadministration is unavoidable, monitor for increased risk of adverse reactions.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], Pralatrexate injection can cause fetal harm when administered to a pregnant woman. There are insufficient data on Pralatrexate injection use in pregnant women to evaluate for a drug- associated risk. Pralatrexate injection was embryotoxic and fetotoxic in rats and rabbits when administered during organogenesis at doses about 1.2% (0.012 times) of the clinical dose on a mg/m2 basis. Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Pralatrexate was embryotoxic and fetotoxic in rats at intravenous doses of 0.06 mg/kg/day (0.36 mg/m2/day or about 1.2% of the clinical dose on a mg/m2 basis) given on gestation days 7 through 20. Treatment with pralatrexate caused a dose-dependent decrease in fetal viability manifested as an increase in late, early, and total resorptions. There was also a dosedependent increase in post-implantation loss. In rabbits, intravenous doses of 0.03 mg/kg/day (0.36 mg/m2/day) or greater given on gestation days 8 through 21 also caused abortion and fetal lethality. This toxicity manifested as early and total resorptions, post-implantation loss, and a decrease in the total number of live fetuses.
8.2 Lactation
Risk Summary
There is no data on the presence of pralatrexate in human milk or its effects on the breastfed child or milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with Pralatrexate injection and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
Pralatrexate injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiation of Pralatrexate injection.
8.4 Pediatric Use
The safety and effectiveness of Pralatrexate injection in pediatric patients have not been established.
8.5 Geriatric Use
In the Study PDX-008, 36% of patients (n = 40) were 65 years of age and over. No overall differences in efficacy and safety were observed in patients based on age (< 65 years compared with ≥ 65 years). Due to the contribution of renal excretion to overall clearance of pralatrexate (approximately 34%), age-related decline in renal function may lead to a reduction in clearance and a commensurate increase in plasma exposure. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Since elderly patients may be at higher risk, monitor more closely. Omit dose and subsequently adjust or discontinue therapy for adverse reactions [see Dosage and Administration (2.4)].
8.6 Renal Impairment
No dosage modification is recommended for patients with mild or moderate renal impairment (eGFR 30 to 59 mL/min/1.73 m2 based on MDRD). For patients with severe renal impairment (eGFR 15 to 29 mL/min/1.73 m2), reduce the recommended dose of Pralatrexate injection [see Dosage and Administration (2.3)].
Serious adverse drug reactions, including TEN and mucositis, have been reported in patients with ESRD undergoing dialysis. Avoid the use of Pralatrexate injection in patients with ESRD with or without dialysis. If the potential benefit of administration justifies the potential risk, monitor renal function and reduce the Pralatrexate injection dose based on adverse reactions [see Dosage and Administration (2.3), Warnings and Precautions (5.6)].
-
10 OVERDOSAGE
No specific information is available on the treatment of overdosage of Pralatrexate injection. If an overdose occurs, general supportive measures should be instituted as deemed necessary by the treating healthcare provider. Based on Pralatrexate injection's mechanism of action, consider the prompt administration of leucovorin.
-
11 DESCRIPTION
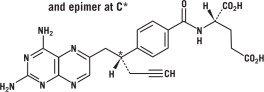
Pralatrexate is a dihydrofolate reductase inhibitor. Pralatrexate has the chemical name (2S)-2[[4-[(1RS)-1-[(2, 4-diaminopteridin-6-yl)methyl]but-3- ynyl]benzoyl]amino]pentanedioic acid. The molecular formula is C23H23N7O5 and the molecular weight is 477.48 g/mol. Pralatrexate is a 1:1 racemic mixture of S- and R- diastereomers at the C10 position (indicated with *). The structural formula is as follows:
Pralatrexate is an off-white to yellow solid. It is soluble in aqueous solutions at pH 6.5 or higher. Pralatrexate is practically insoluble in chloroform and ethanol. The pKa values are 3.25, 4.76, and 6.17.
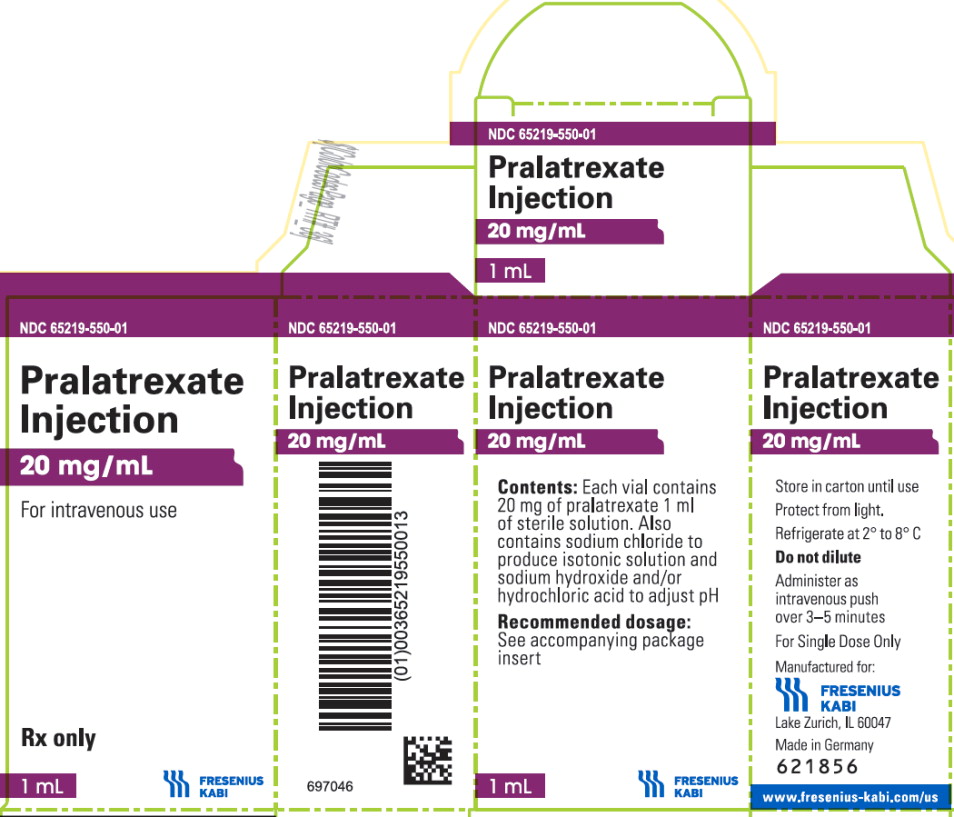
Pralatrexate Injection is supplied as a preservative-free, sterile, isotonic, non-pyrogenic clear yellow aqueous solution contained in a clear glass single-dose vial (Type I) for intravenous use. Each 1 mL of solution contains 20 mg of pralatrexate, sufficient sodium chloride to achieve an isotonic (280-300 mOsm) solution, and sufficient sodium hydroxide, and hydrochloric acid if needed, to adjust and maintain the pH at 7.5-8.5. Pralatrexate Injection is supplied as either 20 mg (1 mL) or 40 mg (2 mL) single-dose vials at a concentration of 20 mg/mL.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pralatrexate is a folate analog metabolic inhibitor that competitively inhibits dihydrofolate reductase. It is also a competitive inhibitor for polyglutamylation by the enzyme folylpolyglutamyl synthetase. This inhibition results in the depletion of thymidine and other biological molecules the synthesis of which depends on single carbon transfer.
12.2 Pharmacodynamics
Pralatrexate exposure-response relationship and the time course of pharmacodynamics responses are unknown.
12.3 Pharmacokinetics
Pralatrexate is a racemic mixture of S- and R-diastereomers. The pharmacokinetics of pralatrexate at the recommended dosage of 30 mg/m2 once weekly have been evaluated in 10 patients with PTCL. Pralatrexate total systemic exposure (AUC) and maximum plasma concentration (Cmax) increased proportionally over a dose range 30 to 325 mg/m2 (10.8 times the approved recommended dosage). No accumulation of pralatrexate was observed.
Distribution
Steady-state volume of distribution of pralatrexate S- and R-diastereomers is 105 L and 37 L, respectively. Protein binding of pralatrexate is approximately 67% in vitro.
Elimination
The total systemic clearance of pralatrexate diastereomers was 417 mL/min (S-diastereomer) and 191 mL/min (R-diastereomer). The terminal elimination half-life of pralatrexate was 12-18 hours (coefficient of variance [CV] = 62-120%).
Metabolism
Pralatrexate is not significantly metabolized by CYP450 isozymes or glucuronidases in vitro.
Excretion
Following a single dose of Pralatrexate injection 30 mg/m2, approximately 34% of the pralatrexate dose was excreted unchanged into urine. Following a radiolabeled pralatrexate dose, 39% (CV = 28%) of the dose was recovered in urine as unchanged pralatrexate and 34% (CV = 88%) in feces as unchanged pralatrexate and/or any metabolites. 10% (CV = 95%) of the dose was exhaled over 24 hours.
Specific Populations
No clinically meaningful effect on the pharmacokinetics of pralatrexate was observed based on sex.
The effect of hepatic impairment on the pharmacokinetics of pralatrexate has not been studied.
Patients with Renal Impairment
Following administration of a single dose of Pralatrexate injection, mean exposures of the pralatrexate S-diastereomer and R-diastereomer were comparable in patients with mild to moderate (eGFR 30 to 59 mL/min/1.73 m2 based on MDRD) renal impairment as compared with severe (eGFR 15 to 29 mL/min/1.73 m2) renal impairment. The mean fraction of the administered dose excreted as unchanged diastereomers in urine (fe) decreased with declining renal function [see Use in Specific Populations (8.6)].
Drug Interaction Studies
Clinical Studies
Coadministration of probenecid (an inhibitor of multidrug resistance-associated protein 2 [MRP2] in vitro) resulted in delayed clearance of pralatrexate.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Pralatrexate does not induce or inhibit CYP enzymes.
Transporter Systems: Pralatrexate is a substrate for BCRP, MRP2, MRP3, and OATP1B3, but is not a substrate of P-gp, OATP1B1, OCT2, OAT1, or OAT3.
Pralatrexate inhibits MRP2 and MRP3, but does not inhibit P-gp, BCRP, OCT2, OAT1, OAT3, OATP1B1, or OATP1B3. MRP3 is a transporter that may affect the transport of etoposide and teniposide.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
-
14 CLINICAL STUDIES
The efficacy of Pralatrexate injection was evaluated in Study PDX-008, an open-label, single-arm, multi-center, international trial that enrolled patients with relapsed or refractory PTCL. One hundred and eleven patients received Pralatrexate injection 30 mg/m2 intravenously over 3 to 5 minutes once weekly by for 6 weeks in 7-week cycles until disease progression or unacceptable toxicity. Of the 111 patients treated, 109 patients were evaluable for efficacy. Evaluable patients had histologically confirmed PTCL by independent central review using the Revised European American Lymphoma (REAL) World Health Organization (WHO) disease classification, and relapsed or refractory disease after at least one prior treatment.
The major efficacy outcome measure was overall response rate (complete response, complete response unconfirmed, and partial response) as assessed by International Workshop Criteria (IWC). An additional efficacy outcome measure was duration of response. Response assessments were scheduled at the end of cycle 1 and then every other cycle (every 14 weeks). Duration of response was measured from the first day of documented response to disease progression or death. Response and disease progression were evaluated by independent central review using the IWC.
The median age was 59 years (range: 21 to 85); 68% were male; 72% were White, 13% were Black, 8% were Hispanic and 5% were Asian. Patients had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 (39%), 1 (44%), or 2 (17%). The median time from initial diagnosis to study entry was 1.3 years (range 24 days to 26.8 years). The median number of prior systemic therapies was 3 (range 1 to 12). Approximately 24% of patients (n = 27) did not have evidence of response to any previous therapy. Approximately 63% of patients (n = 70) did not have evidence of response to their most recent prior therapy before entering the study.
Efficacy results are provided in Table 5.
Table 5 Efficacy Results for Study PDX-008 per Independent Central Review (IWC) Fourteen patients went off treatment in cycle 1; 2 patients were unevaluable for response by IWC due to insufficient materials provided to central review.
CR = Complete Response, CRu = Complete Response unconfirmed, PR = Partial Response
Evaluable Patients
(N=109)N (%) 95% CI Median Duration of Response Range of Duration of Response Overall Response CR+CRu+PR 29 (27) 19, 36 287 days (9.4 months) 1-503 days CR/CRu 9 (8) PR 20 (18) Responses ≥ 14 weeks CR+CRu+PR 13 (12) 7, 20 Not Reached 98-503 days CR/CRu 7 (6) PR 6 (6) The initial response assessment was scheduled at the end of cycle 1. Of the responders, 66% responded within cycle 1. The median time to first response was 45 days (range 37-349 days).
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Pralatrexate Injection is available in single-dose clear glass vials containing pralatrexate at a concentration of 20 mg/mL as a preservative-free, sterile, clear yellow solution individually packaged for intravenous use in the following presentations:
Product Code Unit of Sale Strength 550101 NDC: 65219-550-01 20 mg/1 mL 552102 NDC: 65219-552-02 40 mg/2 mL Store refrigerated at 2-8°C (36-46°F) [see USP Controlled Cold Temperature] in original carton to protect from light.
Pralatrexate Injection is a hazardous drug. Follow applicable special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Folic Acid and Vitamin B12 Supplementation
Advise patients treated with Pralatrexate injection to take folic acid and vitamin B12 to reduce the risk of possible side effects [see Dosage and Administration (2.1)].
Myelosuppression
Inform patients of the risk of myelosuppression and to immediately contact their healthcare provider should any signs of infection develop, including fever. Inform patients to contact their healthcare provider if bleeding or symptoms of anemia occur [see Warnings and Precautions (5.1)].
Mucositis
Inform patients of the signs and symptoms of mucositis. Instruct patients on ways to reduce the risk of its development, and on ways to maintain nutrition and control discomfort from mucositis if it occurs [see Warnings and Precautions (5.2)].
Dermatologic Reactions
Advise patients about the risks for and the signs and symptoms of dermatologic reactions. Instruct patients to immediately notify their healthcare provider if any skin reactions occur [see Warnings and Precautions (5.3)].
Tumor Lysis Syndrome
Inform patients about the risk of and the signs and symptoms of tumor lysis syndrome. Patients should be instructed to notify their healthcare provider if they experience these symptoms [see Warnings and Precautions (5.4)].
Concomitant Medications
Patients should be instructed to inform their healthcare provider if they are taking any concomitant medications including prescription drugs (such as trimethoprim/sulfamethoxazole and probenecid) and nonprescription drugs (such as nonsteroidal anti-inflammatory drugs) [see Drug Interactions (7.1)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females or reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1)].
Advise females patients of reproductive potential to use effective contraception during treatment with Pralatrexate injection and for 6 months after the final dose [see Use in Specific Populations (8.3)].
Advise males with female partners of reproductive potential to use effective contraception during treatment with Pralatrexate injection and for at least 3 months after the final dose [see Use in Specific Populations (8.3)]
Lactation
Advise females women not to breastfeed during treatment with Pralatrexate injection and for 1 week after the final dose [see Use in Specific Populations (8.2)].
Manufactured for:
Lake Zurich, IL 60047
www.fresenius-kabi.com/us
Made in Germany
451756
-
PATIENT PACKAGE INSERT
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – Pralatrexate Injection 20 mg/mL – Carton
NDC: 65219-550-01
Pralatrexate
Injection20 mg/mL
For intravenous use
Rx only
1 mL -
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – Pralatrexate Injection 20 mg/mL – Vial Label
NDC: 65219-550-01
Pralatrexate
Injection20 mg/mL
1 mL
Rx only
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – Pralatrexate Injection 40 mg/2 mL – Carton
NDC: 65219-552-02
Pralatrexate
Injection40 mg/2 mL
(20 mg/mL)For intravenous use
1 mL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – Pralatrexate Injection 40 mg/2 mL – Vial Label
NDC: 65219-552-02
Pralatrexate
Injection
40 mg/2 mL
(20 mg/mL)2 mL
Rx only
-
INGREDIENTS AND APPEARANCE
PRALATREXATE
pralatrexate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65219-550 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pralatrexate (UNII: A8Q8I19Q20) (Pralatrexate - UNII:A8Q8I19Q20) Pralatrexate 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65219-550-01 1 in 1 CARTON 11/15/2022 1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA022468 11/15/2022 PRALATREXATE
pralatrexate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65219-552 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pralatrexate (UNII: A8Q8I19Q20) (Pralatrexate - UNII:A8Q8I19Q20) Pralatrexate 40 mg in 2 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65219-552-02 1 in 1 CARTON 11/15/2022 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA022468 11/15/2022 Labeler - Fresenius Kabi USA, LLC (013547657) Registrant - Acrotech Biopharma Inc. (116965616) Establishment Name Address ID/FEI Business Operations Baxter Oncology, GmbH, (Baxter) 344276063 MANUFACTURE(65219-550, 65219-552)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.