LUMIGAN- bimatoprost solution/ drops
LUMIGAN by
Drug Labeling and Warnings
LUMIGAN by is a Prescription medication manufactured, distributed, or labeled by Allergan, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LUMIGAN® 0.01% safely and effectively. See full prescribing information for LUMIGAN® 0.01%.
LUMIGAN® (bimatoprost ophthalmic solution) 0.01% for topical ophthalmic use
Initial U.S. Approval: 2001RECENT MAJOR CHANGES
Contraindications, Hypersensitivity (4) 07/2017
INDICATIONS AND USAGE
LUMIGAN® 0.01% is a prostaglandin analog indicated for the reduction of elevated intraocular pressure in patients with open angle glaucoma or ocular hypertension. (1)
DOSAGE AND ADMINISTRATION
One drop in the affected eye(s) once daily in the evening. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing 0.1 mg/mL bimatoprost. (3)
CONTRAINDICATIONS
Hypersensitivity. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reaction is conjunctival hyperemia (31%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Use in pediatric patients below the age of 16 years is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
5.2 Eyelash Changes
5.3 Intraocular Inflammation
5.4 Macular Edema
5.5 Bacterial Keratitis
5.6 Use with Contact Lenses
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening. LUMIGAN® (bimatoprost ophthalmic solution) 0.01% should not be administered more than once daily since it has been shown that more frequent administration of prostaglandin analogs may decrease the intraocular pressure lowering effect.
Reduction of the intraocular pressure starts approximately 4 hours after the first administration with maximum effect reached within approximately 8 to 12 hours.
LUMIGAN® 0.01% may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
LUMIGAN® 0.01% is contraindicated in patients with hypersensitivity to bimatoprost or to any of the ingredients [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
Bimatoprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid) and eyelashes. Pigmentation is expected to increase as long as bimatoprost is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of bimatoprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with LUMIGAN® (bimatoprost ophthalmic solution) 0.01% can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly [see Patient Counseling Information (17)].
5.2 Eyelash Changes
LUMIGAN® 0.01% may gradually change eyelashes and vellus hair in the treated eye. These changes include increased length, thickness, and number of lashes. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Intraocular Inflammation
Prostaglandin analogs, including bimatoprost, have been reported to cause intraocular inflammation. In addition, because these products may exacerbate inflammation, caution should be used in patients with active intraocular inflammation (e.g., uveitis).
5.4 Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with bimatoprost ophthalmic solution. LUMIGAN® 0.01% should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
5.5 Bacterial Keratitis
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface [see Patient Counseling Information (17)].
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Pigmentation including blepharal pigmentation and iris hyperpigmentation [see Warnings and Precautions (5.1)]
- Eyelash Changes [see Warnings and Precautions (5.2)]
- Intraocular Inflammation [see Warnings and Precautions (5.3)]
- Macular Edema [see Warnings and Precautions (5.4)]
- Hypersensitivity [see Contraindications (4)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a 12-month clinical study with bimatoprost ophthalmic solutions 0.01%, the most common adverse reaction was conjunctival hyperemia (31%). Approximately 1.6% of patients discontinued therapy due to conjunctival hyperemia. Other adverse drug reactions (reported in 1 to 4% of patients) with LUMIGAN® 0.01% in this study included conjunctival edema, conjunctival hemorrhage, eye irritation, eye pain, eye pruritus, erythema of eyelid, eyelids pruritus, growth of eyelashes, hypertrichosis, instillation site irritation, punctate keratitis, skin hyperpigmentation, vision blurred, and visual acuity reduced.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of LUMIGAN® 0.01%. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to LUMIGAN®, or a combination of these factors include: asthma-like symptoms, dry eye, dyspnea, eye discharge, eye edema, foreign body sensation, headache, hypersensitivity including signs and symptoms of eye allergy and allergic dermatitis, lacrimation increased, and periorbital and lid changes including deepening of the eyelid sulcus.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of LUMIGAN® (bimatoprost ophthalmic solution) 0.01% administration in pregnant women. There is no increase in the risk of major birth defects or miscarriages based on bimatoprost postmarketing experience.
In embryofetal developmental studies, administraton of bimatoprost to pregnant mice and rats during organogenesis, resulted in abortion and early delivery at oral doses at least 33 times (mice) or 94 times (rats) the human exposure to bimatoprost 0.03% dosed bilaterally once daily (based on blood area under the curve [AUC] levels). These adverse effects were not observed at 2.6 times (mice) and 47 times (rats) the human exposure to bimatoprost 0.03% dosed bilaterally once daily (based on blood AUC levels).
In pre/postnatal development studies, administration of bimatoprost to pregnant rats from organogenesis to the end of lactation resulted in reduced gestation length and fetal body weight, and increased fetal and pup mortality at oral doses at least 41 times the human systemic exposure to bimatoprost 0.03% dosed bilaterally once daily (based on blood AUC levels). No adverse effects were observed in rat offspring at exposures estimated at 14 times the human exposure to bimatoprost 0.03% dosed bilaterally once daily (based on blood AUC levels).
Because animal reproductive studies are not always predictive of human response LUMIGAN® 0.01% should be administered during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Data
Animal Data
In an embryofetal development rat study, abortion was observed in pregnant rats administered bimatoprost orally during organogenesis at 0.6 mg/kg/day (94 times the human systemic exposure to bimatoprost 0.03% dosed bilaterally once daily, based on AUC). The No Observed Adverse Effect Level (NOAEL) for abortion was 0.3 mg/kg/day (estimated at 47 times the human systemic exposure to bimatoprost 0.03% dosed bilaterally once daily, based on AUC). No abnormalities were observed in rat fetuses at doses up to 0.6 mg/kg/day.
In an embryofetal development mouse study, abortion and early delivery were observed in pregnant mice administered bimatoprost orally during organogenesis at doses greater than or equal to 0.3 mg/kg/day (33 times the human systemic exposure to bimatoprost 0.03% dosed bilaterally once daily, based on AUC). The NOAEL for abortion and early delivery was 0.1 mg/kg/day (2.6 times the human systemic exposure to bimatoprost 0.03% dosed bilaterally once daily, based on AUC). No abnormalities were observed in mouse fetuses at doses up to 0.6 mg/kg/day (72 times the human systemic exposure to bimatoprost 0.03% dosed bilaterally once
daily, based on AUC).
In a pre/postnatal development study, treatment of pregnant rats with bimatoprost orally from gestation day 7 to lactation day 20 resulted in reduced gestation length, increased late resorptions, fetal deaths, and postnatal pup mortality, and reduced pup body weight at doses greater than or equal to 0.3 mg/kg/day. These effects were observed at exposures at least 41 times the human systemic exposure to bimatoprost 0.03% dosed bilaterally once daily, based on AUC. The NOAEL for postnatal development and mating performance of the offspring was 0.1 mg/kg/day (estimated at 14 times the human systemic exposure to bimatoprost 0.03% dosed bilaterally once daily, based on AUC).
8.2 Lactation
Risk Summary
It is not known whether topical ocular treatment with LUMIGAN® 0.01% could result in sufficient systemic absorption to produce detectable quantities in human milk. In animal studies, bimatoprost has been shown to be present in breast milk of lactating rats at an intravenous dose (i.e., 1 mg/kg) 970 times the RHOD (on a mg/rn 2 basis), however, no animal data is available at clinically relevant doses.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for LUMIGAN® 0.01% and any potential adverse effects on the breastfed child from LUMIGAN® 0.01%.
-
10 OVERDOSAGE
No information is available on overdosage in humans. If overdose with LUMIGAN® (bimatoprost ophthalmic solution) 0.01% occurs, treatment should be symptomatic.
In oral (by gavage) mouse and rat studies, doses up to 100 mg/kg/day did not produce any toxicity. This
dose expressed as mg/m2 is at least 210 times higher than the accidental dose of one bottle of LUMIGAN® 0.01% for a 10 kg child.
-
11 DESCRIPTION
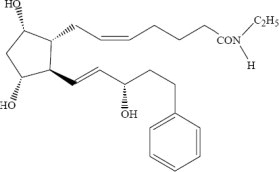
LUMIGAN® (bimatoprost ophthalmic solution) 0.01% is a synthetic prostamide analog with ocular hypotensive activity. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-5-N-ethylheptenamide, and its molecular weight is 415.58. Its molecular formula is C25H37NO4. Its chemical structure is:
Bimatoprost is a powder, which is very soluble in ethyl alcohol and methyl alcohol and slightly soluble in water. LUMIGAN® 0.01% is a clear, isotonic, colorless, sterile ophthalmic solution with an osmolality of approximately 290 mOsmol/kg.
LUMIGAN® 0.01% contains Active: bimatoprost 0.1 mg/mL; Inactives: benzalkonium chloride 0.2 mg/mL; sodium chloride; sodium phosphate, dibasic; citric acid; and purified water. Sodium hydroxide and/or hydrochloric acid may be added to adjust pH. The pH during its shelf life ranges from 6.8-7.8.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bimatoprost, a prostaglandin analog, is a synthetic structural analog of prostaglandin with ocular hypotensive activity. It selectively mimics the effects of naturally occurring substances, prostamides. Bimatoprost is believed to lower intraocular pressure (IOP) in humans by increasing outflow of aqueous humor through both the trabecular meshwork and uveoscleral routes. Elevated IOP presents a major risk factor for glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss.
12.3 Pharmacokinetics
Absorption: After one drop of bimatoprost ophthalmic solution 0.03% was administered once daily to both eyes of 15 healthy subjects for two weeks, blood concentrations peaked within 10 minutes after dosing and were below the lower limit of detection (0.025 ng/mL) in most subjects within 1.5 hours after dosing. Mean Cmax and AUC0-24hr values were similar on days 7 and 14 at approximately 0.08 ng/mL and 0.09 nghr/mL,
respectively, indicating that steady state was reached during the first week of ocular dosing. There was no
significant systemic drug accumulation over time.
Distribution: Bimatoprost is moderately distributed into body tissues with a steady-state volume of distribution of 0.67 L/kg. In human blood, bimatoprost resides mainly in the plasma. Approximately 12% of bimatoprost remains unbound in human plasma.
Metabolism: Bimatoprost is the major circulating species in the blood once it reaches the systemic circulation following ocular dosing. Bimatoprost then undergoes oxidation, N-deethylation and glucuronidation to form a diverse variety of metabolites.
Elimination: Following an intravenous dose of radiolabeled bimatoprost (3.12 mcg/kg) to six healthy subjects, the maximum blood concentration of unchanged drug was 12.2 ng/mL and decreased rapidly with an elimination half-life of approximately 45 minutes. The total blood clearance of bimatoprost was 1.5 L/hr/kg. Up to 67% of the administered dose was excreted in the urine while 25% of the dose was recovered in the feces.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Bimatoprost was not carcinogenic in either mice or rats when administered by oral gavage for 104 weeks at doses up to 2 mg/kg/day and 1 mg/kg/day, respectively (192 and 291 times the estimated human systemic exposure to bimatoprost 0.03% dosed bilaterally once daily, respectively, based on blood AUC levels)
Bimatoprost was not mutagenic or clastogenic in the Ames test, in the mouse lymphoma test, or in the in vivo mouse micronucleus tests.
Bimatoprost did not impair fertility in male or female rats up to doses of 0.6 mg/kg/day (at least 103 times the recommended human exposure to bimatoprost 0.03% dosed bilaterally once daily based on blood AUC levels).
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
LUMIGAN® (bimatoprost ophthalmic solution) 0.01% is supplied sterile in opaque white low density
polyethylene ophthalmic dispenser bottles and tips with turquoise polystyrene caps in the following sizes:
2.5 mL fill in a 5 mL container - NDC: 0023-3205-03
5 mL fill in a 10 mL container - NDC: 0023-3205-05
7.5 mL fill in a 10 mL container - NDC: 0023-3205-08 -
17 PATIENT COUNSELING INFORMATION
Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Also inform patients about the possibility of eyelid skin darkening, which may be reversible after discontinuation of LUMIGAN® (bimatoprost ophthalmic solution) 0.01%.
Potential for Eyelash Changes
Inform patients of the possibility of eyelash and vellus hair changes in the treated eye during treatment with LUMIGAN® 0.01%. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
Handling the Container
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye,
surrounding structures, fingers, or any other surface in order to avoid contamination of the solution by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
When to Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection), have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician's advice concerning the continued use of LUMIGAN® 0.01%.
Use with Contact Lenses
Advise patients that LUMIGAN® 0.01% contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of LUMIGAN® 0.01% and may be reinserted 15 minutes following its administration.
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes between applications.
© 2017 Allergan. All rights reserved.
All trademarks are the property of their respective owners.
Patented. See: www.allergan.com/patents
Irvine, CA 92612
Made in the U.S.A.71807US15
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LUMIGAN
bimatoprost solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0023-3205 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bimatoprost (UNII: QXS94885MZ) (bimatoprost - UNII:QXS94885MZ) bimatoprost 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) benzalkonium chloride (UNII: F5UM2KM3W7) sodium phosphate, dibasic (UNII: GR686LBA74) citric acid monohydrate (UNII: 2968PHW8QP) water (UNII: 059QF0KO0R) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-3205-02 1 in 1 CARTON 09/10/2010 1 2.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 0023-3205-03 1 in 1 CARTON 09/10/2010 2 2.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 3 NDC: 0023-3205-05 1 in 1 CARTON 09/10/2010 3 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 4 NDC: 0023-3205-08 1 in 1 CARTON 09/10/2010 4 7.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022184 09/10/2010 Labeler - Allergan, Inc. (144796497) Establishment Name Address ID/FEI Business Operations Allergan, Inc. 362898611 MANUFACTURE(0023-3205) Establishment Name Address ID/FEI Business Operations Allergan Pharmaceuticals Ireland 219682291 MANUFACTURE(0023-3205)
Trademark Results [LUMIGAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LUMIGAN 78054980 2604942 Live/Registered |
ALLERGAN, INC. 2001-03-26 |
 LUMIGAN 75548965 2494680 Live/Registered |
ALLERGAN, INC. 1998-09-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.