PECGEN DMX- dextromethorphan hbr, guaifenesin solution
PECGEN DMX by
Drug Labeling and Warnings
PECGEN DMX by is a Otc medication manufactured, distributed, or labeled by KRAMER NOVIS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active Ingredients (in each 5 mL tsp)
- Purposes
- Uses
-
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
cough that occurs with too much phlegm (mucus)
a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema.When using this product, do not use more than directed.
Stop use and ask a doctor if
symptoms do not get better within 7 days or are accompanied by fever
cough lasts more than 7 days, comes back, or is accompanied by fever, rash or persistent headache. These could be signs of a serious condition.If pregnant or breast-feeding, ask a health professional before use.
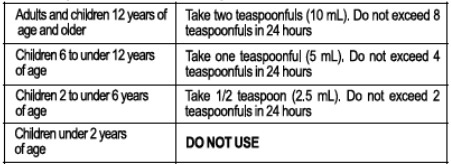
- Directions
- Inactive ingredients
- Other information
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
Contains the same active ingredients as Trispec® DMX*
Sugar, Alcohol, Dye and Gluten FREE
CHERRY RASPBERRY FLAVOR
Manufactured in the USA for Kramer Novis
*Trispec® DMX is a registered trademark of Deliz Pharmaceutical Corp. This product is not manufactured, distributed or marketed by Deliz Pharmaceutical Corp.
- Packaging
-
INGREDIENTS AND APPEARANCE
PECGEN DMX
dextromethorphan hbr, guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 52083-630 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 187 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor CHERRY, RASPBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52083-630-16 474 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 04/01/2015 Labeler - KRAMER NOVIS (090158395)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.