CITANEST FORTE- prilocaine hydrochloride and epinephrine bitartrate injection, solution
Citanest Forte by
Drug Labeling and Warnings
Citanest Forte by is a Prescription medication manufactured, distributed, or labeled by Dentsply Pharmaceutical Inc., Novocol. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Citanest Dental (prilocaine HCl) Injection is a sterile, non pyrogenic isotonic solution that contains a local anesthetic agent with or without epinephrine (as bitartrate) and is administered parenterally by injection. See INDICATIONS AND USAGE for specific uses. The quantitative composition of each available injection is shown in Table 1.
Citanest Dental injections contain prilocaine HCl, which is chemically designated as propanamide, N-(2-methyl-phenyl) -2- (propylamino)-, monohydrochloride and has the following structural formula:
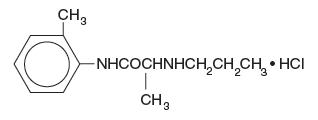
C 13H 20N 2o ∙ HCl molecular wt = 256.77
Epinephrine is (-) -3, 4-Dihydroxy-a-[(methylamino) methyl] benzyl alcohol and has the following structural formula:
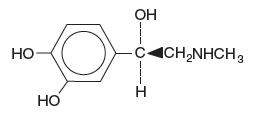
C 9H 13NO 3 molecular wt = 183.21
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
The specific quantitative composition of each available injection is shown in Table 1.
TABLE 1. COMPOSITION OF AVAILABLE INJECTIONS Product Identification Formula (mg/mL) Prilocaine HCL Epinephrine
(as the bitartrate)Citric Acid Sodium Metabisulfate ph Citanest Plain Dental Injection 40.0 None None None 6.0-7.0 Citanest Forte Dental Injection with Epinephrine 40.0 0.005 0.2 0.5 3.3-5.5 Note: Sodium hydroxide and/or hydrochloric acid may be used to adjust the pH of Citanest Dental Injections. Filled under nitrogen.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Prilocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Onset and Duration of Action
When used for infiltration injection in dental patients, the time of onset of anesthesia with Citanest Plain Dental Injection and Citanest Forte Dental Injection averages less than 2 minutes with an average duration of soft tissue anesthesia of approximately 2 hours with Citanest Plain Dental Injection and approximately 2¼ hours with Citanest Forte Dental Injection.
Based on electrical stimulation studies, Citanest Plain Dental Injection provides a duration of pulpal anesthesia of approximately 10 minutes in maxillary infiltration injections. In clinical studies, this has been found to provide complete anesthesia for procedures lasting an average of 20 minutes.
When used for inferior alveolar nerve block, the time of onset of Citanest Plain Dental Injection and Citanest Forte Dental Injection averages less than three minutes with an average duration of soft tissue anesthesia of approximately 2½ hours with Citanest Plain Dental Injection and approximately 3 hours with Citanest Forte Dental Injection.
Hemodynamics
Excessive blood levels may cause changes in cardiac output, total peripheral resistance, and mean arterial pressure. These changes may be attributable to a direct depressant effect of the local anesthetic agent on various components of the cardiovascular system and/or the beta-adrenergic receptor stimulating action of epinephrine when present.
Pharmacokinetics and Metabolism
Information derived from diverse formulations, concentrations and usages reveals that prilocaine is completely absorbed following parenteral administration, its rate of absorption depending, for example, upon such factors as the site of administration and the presence or absence of a vasoconstrictor agent. Prilocaine is metabolized in both the liver and the kidney and excreted via the kidney. It is not metabolized by plasma esterases. Hydrolysis of prilocaine by amidases yields ortho-toluidine and N-propylalanine. Both of these compounds may undergo ring hydroxylation.
O-toluidine has been found to produce methemoglobin, both in vitro and in vivo (see ADVERSE REACTIONS).
Because prilocaine is metabolized in both the liver and kidneys, hepatic and renal dysfunction may alter prilocaine kinetics.
As with other local anesthetic agents, the plasma binding of prilocaine may be dependent on drug concentration. At 0.5–1.0 mg/mL it is 55% protein bound.
Prilocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of prilocaine required to produce overt systemic effects. In the rhesus monkey, arterial blood levels of 20 mg/mL have been shown to be the threshold for convulsive activity.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
DENTAL PRACTITIONERS WHO EMPLOY LOCAL ANESTHETIC AGENTS SHOULD BE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF EMERGENCIES THAT MAY ARISE FROM THEIR USE. RESUSCITATIVE EQUIPMENT, OXYGEN AND OTHER RESUSCITATIVE DRUGS SHOULD BE AVAILABLE FOR IMMEDIATE USE.
To minimize the likelihood of intravascular injection, aspiration should be performed before the local anesthetic solution is injected. If blood is aspirated, the needle must be repositioned until no return of blood can be elicited by aspiration. Note, however, that the absence of blood in the syringe does not assure that intravascular injection will be avoided.
Citanest Dental with epinephrine injections contain sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Methemoglobinemia
Prilocaine has been associated with the development of methemoglobinemia. Very young patients, patients with congenital or idiopathic methemoglobinemia, or patients with glucose-6-phosphate deficiencies are more susceptible to methemoglobinemia.
Patients taking drugs associated with drug induced methemoglobinemia such as sulfonamides, acetaminophen, acetanilid, aniline dyes, benzocaine, chloroquine, dapsone, naphthalene, nitrates and nitrites, nitrofurantoin, nitroglycerin, nitroprusside, pamaquine, para-aminosalicylic acid, phenacetin, phenobarbital, phenytoin, primaquine, and quinine are also at greater risk for developing methemoglobinemia.
-
PRECAUTIONS
General
The safety and effectiveness of prilocaine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be consulted for specific techniques and precautions for various regional anesthetic procedures. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use. (See WARNINGS and ADVERSE REACTIONS.) The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Repeated doses of prilocaine may cause significant increases in blood levels with each repeated dose because of slow accumulation of the drug or its metabolites. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients, acutely ill patients, and children should be given reduced doses commensurate with their age and physical status. Prilocaine should also be used with caution in patients with severe shock or heart block.
Local anesthetic injections containing a vasoconstrictor should be used cautiously in areas of the body supplied by end arteries or having otherwise compromised blood supply. Patients with peripheral vascular disease and those with hypertensive vascular disease may exhibit exaggerated vasoconstrictor response. Ischemic injury or necrosis may result. Preparations containing a vasoconstrictor should be used with caution in patients during or following the administration of potent general anesthetic agents, since cardiac arrhythmias may occur under such conditions.
Cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness should be monitored after each local anesthetic injection. Restlessness, anxiety, tinnitus, dizziness, blurred vision, tremors, depression or drowsiness should alert the practitioner to the possibility of central nervous system toxicity. Signs and symptoms of depressed cardiovascular function may commonly result from a vasovagal reaction, particularly if the patient is in an upright position. (See ADVERSE REACTIONS, Cardiovascular System.)
Since amide-type local anesthetics are metabolized by the liver, prilocaine should be used with caution in patients with hepatic disease.
Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at greater risk of developing toxic plasma concentrations. Prilocaine should also be used with caution in patients with impaired cardiovascular function since they may be less able to compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
Many drugs used during the conduct of anesthesia are considered potential triggering agents for familial malignant hyperthermia. Since it is not known whether amide-type local anesthetics may trigger this reaction and since the need for supplemental general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for the management of malignant hyperthermia should be available. Early unexplained signs of tachycardia, tachypnea, labile blood pressure and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the suspect triggering agent(s) and institution of treatment, including oxygen therapy, indicated supportive measures and dantrolene (consult dantrolene sodium intravenous package insert before using).
Prilocaine should be used with caution in persons with known drug sensitivities. Patients allergic to para-aminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to prilocaine.
Use in the Head and Neck Area
Small doses of local anesthetics injected into the head and neck area, including retrobulbar, dental and stellate ganglion blocks, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Confusion, convulsions, respiratory depression and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. Patients receiving these blocks should have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded. (See DOSAGE AND ADMINISTRATION.)
Information for Patients
The patient should be informed of the possibility of temporary loss of sensation and muscle function following infiltration or nerve block injections.
The patient should be advised to exert caution to avoid inadvertent trauma to the lips, tongue, cheek mucosae or soft palate when these structures are anesthetized. The ingestion of food should therefore be postponed until normal function returns.
The patient should be advised to consult the dentist if anesthesia persists, or if a rash develops.
Clinically Significant Drug Interactions
The administration of local anesthetic injections containing epinephrine or norepinephrine to patients receiving monoamine oxidase inhibitors, tricyclic antidepressants or phenothiazines may produce severe, prolonged hypotension or hypertension. Concurrent use of these agents should generally be avoided. In situations when concurrent therapy is necessary, careful patient monitoring is essential.
Concurrent administration of vasopressor drugs and ergot-type oxytocic drugs may cause severe, persistent hypertension or cerebrovascular accidents.
Prilocaine may contribute to the formation of methemoglobinemia in patients treated with other drugs known to cause this condition (see methemoglobinemia subsection of WARNINGS).
Drug/Laboratory Test Interactions
The intramuscular injection of prilocaine may result in an increase in creatine phosphokinase levels. Thus, the use of this enzyme determination, without isoenzyme separation, as a diagnostic test for the presence of acute myocardial infarction may be compromised by the intramuscular injection of prilocaine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of prilocaine in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
Chronic oral toxicity studies of ortho-toluidine, a metabolite of prilocaine, in mice (150–4800 mg/kg) and rats (150–800 mg/kg) have shown that ortho-toluidine is a carcinogen in both species. The lowest dose corresponds to approximately 50 times the maximum amount of ortho-toluidine to which a 50 kg subject would be expected to be exposed following a single injection (8 mg/kg) of prilocaine.
Ortho-toluidine (0.5 mg/mL) showed positive results in Escherichia coli DNA repair and phage-induction assays. Urine concentrates from rats treated with ortho-toluidine (300 mg/kg, orally) were mutagenic for Salmonella typhimurium with metabolic activation. Several other tests, including reverse mutations in five different Salmonella typhimurium strains with or without metabolic activation and single strand breaks in DNA of V79 Chinese hamster cells, were negative.
Use in Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed in rats at doses up to 30 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to prilocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering prilocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when prilocaine is administered to a nursing woman.
Pediatric Use
Dosages in children should be reduced, commensurate with age, body weight, and physical condition. (See DOSAGE AND ADMINISTRATION.)
-
ADVERSE REACTIONS
Swelling and persistent paresthesia of the lips and oral tissues may occur. Persistent paresthesia lasting weeks to months, and in rare instances paresthesia lasting greater than one year have been reported.
Adverse experiences following the administration of prilocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage, rapid absorption or unintentional intravascular injection, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
Central Nervous System
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression, and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest.
Drowsiness following the administration of prilocaine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular System
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Signs and symptoms of depressed cardiovascular function may commonly result from a vasovagal reaction, particularly if the patient is in an upright position. Less commonly, they may result from a direct effect of the drug. Failure to recognize the premonitory signs such as sweating, a feeling of faintness, changes in pulse or sensorium may result in progressive cerebral hypoxia and seizure or serious cardiovascular catastrophe. Management consists of placing the patient in the recumbent position and ventilation with oxygen. Supportive treatment of circulatory depression may require the administration of intravenous fluids, and, when appropriate, a vasopressor (eg, ephedrine) as directed by the clinical situation.
Allergic
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions as a result of sensitivity to prilocaine are extremely rare and, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
Neurologic
The incidences of adverse reactions (eg, persistent neurologic deficit) associated with the use of local anesthetics may be related to the technique employed, the total dose of local anesthetic administered, the particular drug used, the route of administration, and the physical condition of the patient.
-
OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics (see ADVERSE REACTIONS, WARNINGS, and PRECAUTIONS).
Management of Local Anesthetic Emergencies
The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient's state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
The first step in the management of convulsions consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a benzodiazepine (such as diazepam) may be administered intravenously. The clinician should be familiar, prior to use of local anesthetics, with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor as directed by the clinical situation (eg, ephedrine).
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and cardiac arrest. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated, after initial administration of oxygen by mask, if difficulty is encountered in the maintenance of a patent airway or if prolonged ventilatory support (assisted or controlled) is indicated.
Dialysis is of negligible value in the treatment of acute overdosage with prilocaine.
The development of methemoglobinemia is generally dose related but may occur at any dose in susceptible individuals. While methemoglobin values of less than 20% do not generally produce any clinical symptoms, the appearance of cyanosis at 2–4 hours following administration should be evaluated in terms of the general health status of the patient.
Methemoglobinemia can be reversed when indicated by intravenous administration of methylene blue at a dosage of 1–2 mg/kg given over a five minute period.
The subcutaneous LD 50 of prilocaine HCl in female mice is 550 (359–905) mg/kg.
-
DOSAGE AND ADMINISTRATION
The dosage of Citanest Plain Dental Injection and Citanest Forte Dental Injection varies and depends on the physical status of the patient, the area of the oral cavity to be anesthetized, the vascularity of the oral tissues, and the technique of anesthesia. The least volume of injection that results in effective local anesthesia should be administered. For specific techniques and procedures of local anesthesia in the oral cavity, refer to standard textbooks.
Inferior Alveolar Block
There are no practical clinical differences between Citanest Dental with and without epinephrine when used for inferior alveolar blocks.
Maxillary Infiltration
Citanest Plain Dental is recommended for use in maxillary infiltration anesthesia for procedures in which the painful aspects can be completed within 15 minutes after the injection. Citanest Plain Dental is therefore especially suited to short procedures in the maxillary anterior teeth. For long procedures, or those involving maxillary posterior teeth where soft tissue numbness is not troublesome to the patient, Citanest Forte Dental is recommended.
For most routine procedures, initial dosages of 1 to 2 mL of Citanest Plain Dental Injection or Citanest Forte Dental Injection will usually provide adequate infiltration or major nerve block anesthesia.
The maximum recommended dose that should ever be administered within a two hour period in normal healthy adults should be calculated based upon the patient's weight as follows:
Weight Maximum recommended dose <150 lbs
(<70 kg)4 mg/lb
(8 mg/kg)≥150 lbs
(≥70 kg)600 mg (15 mL) or
8 cartridgesIn children under 10 years of age it is rarely necessary to administer more than one-half cartridge (40 mg) of Citanest Plain Dental Injection or Citanest Forte Dental Injection per procedure to achieve local anesthesia for a procedure involving a single tooth. In maxillary infiltration, this amount will often suffice to the treatment of two or even three teeth. In the mandibular block, however, satisfactory anesthesia achieved with this amount of drug will allow treatment of the teeth in an entire quadrant
ASPIRATION PRIOR TO INJECTION IS RECOMMENDED, since it reduces the possibility of intra-vascular injection, thereby keeping the incidence of side effects and anesthetic failure to a minimum.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit. Solutions that are discolored and/or contain particulate matter should not be used.
Any unused portion of a cartridge of Citanest Plain Dental or Citanest Forte Dental Injection should be discarded.
Maximum Recommended Dosages
In patients weighing <150 lbs (70 kg), no more than 4 mg/lb (8 mg/kg) should be administered. In patients weighing ≥150 lbs, no more than 600 mg (8 cartridges) of prilocaine HCl should be administered as a single injection.
Children
It is difficult to recommend a maximum dose of any drug for children since this varies as a function of age and weight. For children of less than ten years who have a normal lean body mass and normal body development, the maximum dose may be determined by the application of one of the standard pediatric drug formulas (eg, Clark's rule). For example, in a child of five years weighing 50 lbs., the dose of prilocaine hydrochloride should not exceed 150–200 mg (6.6–8.8 mg/kg or 3–4 mg/lb of body weight) when calculated according to Clark's rule.
-
HOW SUPPLIED
4% Citanest Plain Dental Injection (NDC: 66312-520-14 or 66312-520-16) and 4% Citanest Forte Dental Injection with epinephrine 1:200,000 (NDC: 66312-540-14 or 66312-540-16) are dispensed in 1.7 mL cartridges, packed 50 or 100 per box. Not all pack sizes may be marketed.
Sterilization, Storage and Technical Procedures:
- Cartridges of Citanest Plain Dental Injection and Citanest Forte Dental Injection should not be autoclaved, because solutions of epinephrine and the closures employed in cartridges cannot withstand autoclaving temperatures and pressures.
- If chemical disinfection of anesthetic cartridges is desired, either 91% isopropyl alcohol or 70% ethyl alcohol is recommended. Many commercially available brands of rubbing alcohol, as well as solutions of ethyl alcohol not of U.S.P. grade, contain denaturants that are injurious to rubber and, therefore, are not to be used. It is recommended that chemical disinfection be accomplished by wiping the cartridge cap thoroughly with a pledget of cotton that has been moistened with the recommended alcohol just prior to use. IMMERSION IS NOT RECOMMENDED.
- Certain metallic ions (mercury, zinc, copper, etc.) have been related to swelling and edema after local anesthesia in dentistry. Therefore, chemical disinfectants containing or releasing these ions are not recommended. Antirust tablets usually contain metal ions. Accordingly, aluminum sealed cartridges should not be kept in such solutions.
- Quaternary ammonium salts, such as benzalkonium chloride, are electrolytically incompatible with aluminum. Cartridges of Citanest Plain Dental Injection and Citanest Forte Dental Injection are sealed with aluminum caps and therefore should not be immersed in any solution containing these salts.
- To avoid leakage of solutions during injection, be sure to penetrate the center of the rubber diaphragm when loading the syringe. An off-center penetration produces an oval shaped puncture that allows leakage around the needle.
Other causes of leakage and breakage include badly worn syringes, aspirating syringes with bent harpoons, the use of syringes not designed to take 1.7 mL cartridges, and inadvertent freezing.
- Cracking of glass cartridges is most often the result of an attempt to use a cartridge with an extruded plunger. An extruded plunger loses its lubrication and can be forced back into the cartridge only with difficulty. Cartridges with extruded plungers should be discarded.
- Store at room temperature, 25°C (77°F) or below. Do not freeze.
- Solutions containing epinephrine should be protected from light.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 40 mg/mL Cartridge Carton
NDC: 66312-540-16
DENTSPLY
PHARMACEUTICAL4 % Citanest ® Forte DENTAL
with epinephrine 1:200,000(prilocaine HCl and epinephrine
injection, USP) 40 mg/mLCOLOR
CODEDFor dental block and infiltration injections only.
Store at room temperature,
25°C (77°F) or below.
DO NOT PERMIT TO FREEZE.
Rx only
50 Cartridges, 1.7 mL eachSTERILE AQUEOUS
SOLUTION FOR INJECTIONDENTSPLY Reorder #: 48816

-
INGREDIENTS AND APPEARANCE
CITANEST FORTE
prilocaine hydrochloride and epinephrine bitartrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66312-540 Route of Administration SUBMUCOSAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRILOCAINE HYDROCHLORIDE (UNII: MJW015BAPH) (PRILOCAINE - UNII:046O35D44R) PRILOCAINE HYDROCHLORIDE 40 mg in 1 mL EPINEPHRINE BITARTRATE (UNII: 30Q7KI53AK) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.005 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.2 mg in 1 mL SODIUM METABISULFITE (UNII: 4VON5FNS3C) 0.5 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66312-540-14 100 in 1 CARTON 11/18/1965 1 1.7 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 2 NDC: 66312-540-16 50 in 1 CARTON 11/18/1965 2 1.7 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021383 11/18/1965 Labeler - Dentsply Pharmaceutical Inc. (102221942) Establishment Name Address ID/FEI Business Operations Novocol 201719960 manufacture(66312-540)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.