ZINXATION SUNSCREEN SPF-50- zinc oxide lotion
DR ZINX MINERAL FACIAL SUNSCREEN - ACNE PRONE SKIN by
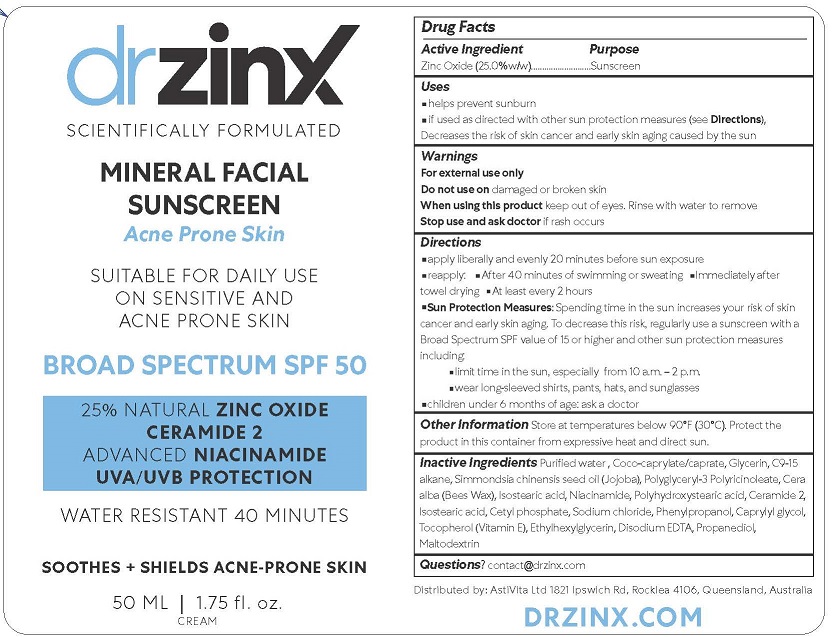
Drug Labeling and Warnings
DR ZINX MINERAL FACIAL SUNSCREEN - ACNE PRONE SKIN by is a Otc medication manufactured, distributed, or labeled by ANTARIA PTY LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
-
DIRECTIONS
apply liberally and evenly 20 minutes before sun exposure
reapply: After 40 minutes of swimming or sweating Immediately after towel drying at least every 2 hoursSun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
children under 6 months of age: ask a doctor -
INACTIVE INGREDIENTS
Purified water, Coco-caprylate/caprate, Glycerin, C9-15 alkane, Simmondsia chinensis seed oil (Jojoba), Polyglyceryl-3 Polyricinoleate, Cera alba (Bees Wax), Isostearic acid, Niacinamide, Polyhydroxystearic acid, Ceramide 2, Isostearic acid, Cetyl phosphate, Sodium chloride, Phenylpropanol, Caprylyl glycol, Tocopherol (Vitamin E), Ethylhexylglycerin, Disodium EDTA, Propanediol, Maltodextrin
- QUESTIONS OR COMMENTS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZINXATION SUNSCREEN SPF-50
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 60396-203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 25 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) GLYCERIN (UNII: PDC6A3C0OX) C13-15 ALKANE (UNII: 114P5I43UJ) JOJOBA OIL (UNII: 724GKU717M) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) YELLOW WAX (UNII: 2ZA36H0S2V) ISOSTEARIC ACID (UNII: X33R8U0062) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) CERAMIDE 2 (UNII: C04977SRJ5) CETYL PHOSPHATE (UNII: VT07D6X67O) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENYLPROPANOL (UNII: 0F897O3O4M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPANEDIOL (UNII: 5965N8W85T) MALTODEXTRIN (UNII: 7CVR7L4A2D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60396-203-11 50 mL in 1 TUBE; Type 0: Not a Combination Product 05/23/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/23/2020 Labeler - ANTARIA PTY LTD (740611405) Establishment Name Address ID/FEI Business Operations ANTARIA PTY LTD 740611405 manufacture(60396-203)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.