VIONEX- chloroxylenol gel
VioNex by
Drug Labeling and Warnings
VioNex by is a Otc medication manufactured, distributed, or labeled by Metrex Research, LLC, Metrex Research. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
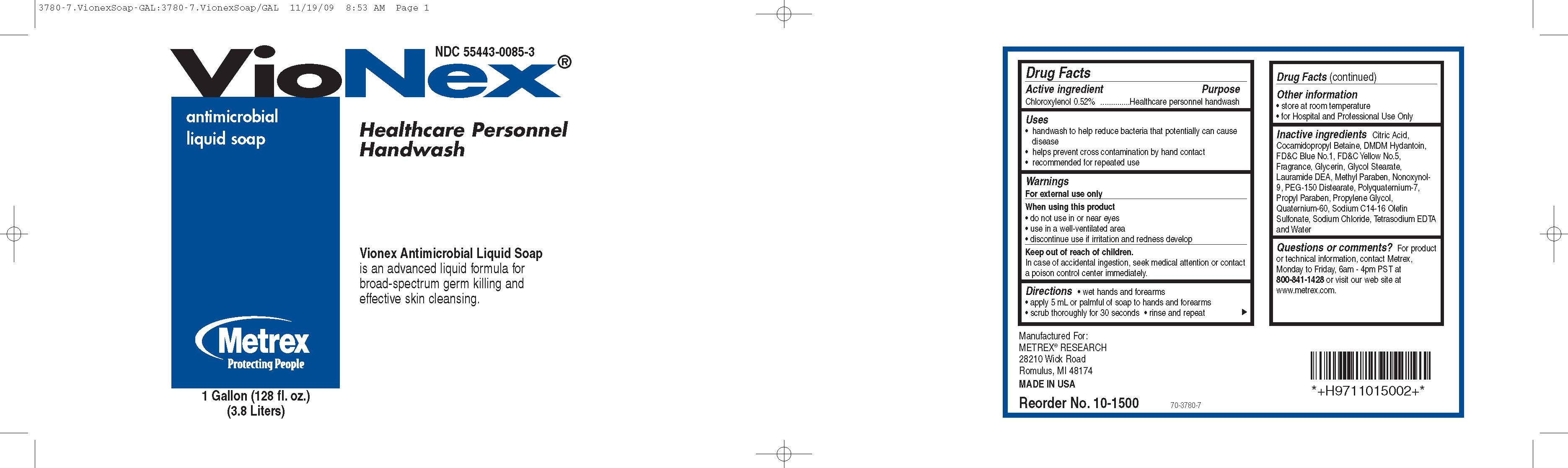
Drug Facts
 Active Ingredients Purpose
Active Ingredients Purpose
Chloroxylenol 0.52%..............................Healthcare personnel handwash
Uses
- handwash to help reduce bacteria that potentially can cause disease
- helps prevent cross contamination by hand contact
- recommended for repeated use
When using this product
- do not use in or near eyes
- use in a well-ventilated area
- discontinue use if irritation and redness develop
Keep out of reach of children
In case of accidental ingestion, seek medical attention or contact a poison control center immediately.
Directions
- wet hands and forearms
- apply 5 mL or palmful of soap to hands and forearms
- scrub thoroughly for 30 seconds
- rinse and repeat
Inactive Ingredients
Citric Acid, Cocamidopropyl Betaine, DMDM Hydantoin, F DC Blue No.1, FD C Yellow No.5, Fragrance, Glycerin, Glycol Stearate, Lauramide DEA, Methyl Paraben, Nonoxynol-9, PEG- 150 Distearate, Polyquaternium-7, Propyl Paraben, Propylene Glycol, Quaternium-60, Sodium C14-16 Olefin Sulfonate, Sodium Chloride, Tetrasodium EDTA and Water
Questions or comments?
For product or technical informaiton, contact Metrex, Monday to Friday, 6am - 4pm PST at 800-841-1428 or visit our website at www.metrex.com.
-
Principal Display Panel
 VioNex antimicrobial liquid soap
VioNex antimicrobial liquid soap
Healthcare Personnel Handwash
VioNex antimicrobial liquid soap is an advanced liquid formula for broad-spectrum germ killing and effective skin cleansing.
Manufactured for:
METREX RESEARCH
28210 Wick Road
Romulus, Michigan 48174
MADE IN USA
Reorder No.:
Metrex
Protecting People
fl. oz. ( mL)
gallon (fl. oz.)
( Liters)
For use in Wall Mount Dispenser (800 mL package only) - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VIONEX
chloroxylenol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55443-0085 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.52 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) PROPYLPARABEN (UNII: Z8IX2SC1OH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CHLORIDE (UNII: 451W47IQ8X) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600 KD) (UNII: 0L414VCS5Y) NONOXYNOL-9 (UNII: 48Q180SH9T) DMDM HYDANTOIN (UNII: BYR0546TOW) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) GLYCERIN (UNII: PDC6A3C0OX) GLYCOL STEARATE (UNII: 0324G66D0E) LAURIC DIETHANOLAMIDE (UNII: I29I2VHG38) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) EDETATE SODIUM (UNII: MP1J8420LU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55443-0085-6 24 in 1 BOX 09/10/2019 1 NDC: 55443-0085-5 118.3 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 55443-0085-1 12 in 1 BOX 09/10/2019 2 NDC: 55443-0085-2 532.3 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC: 55443-0085-4 4 in 1 BOX 09/10/2019 3 NDC: 55443-0085-3 3785.4 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 01/01/1992 Labeler - Metrex Research (102567567) Registrant - Metrex Research (102567567)
Trademark Results [VioNex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIONEX 79165586 4961985 Live/Registered |
SIRONA Dental Systems GmbH 2015-02-12 |
 VIONEX 74274097 1746754 Live/Registered |
METREX RESEARCH, LLC 1992-05-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.