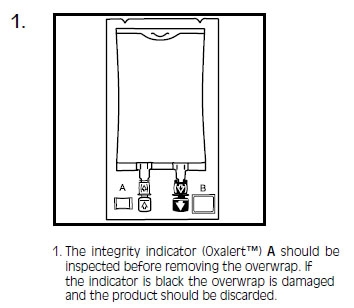
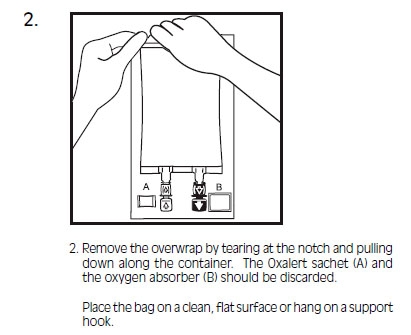
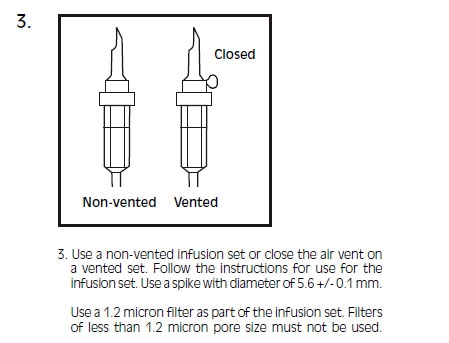
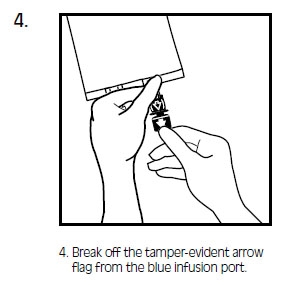
INTRALIPID- i.v. fat emulsion emulsion
Intralipid by
Drug Labeling and Warnings
Intralipid by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation , Fresenius Kabi Deutschland GmbH , Fresenius Kabi AB Uppsala. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
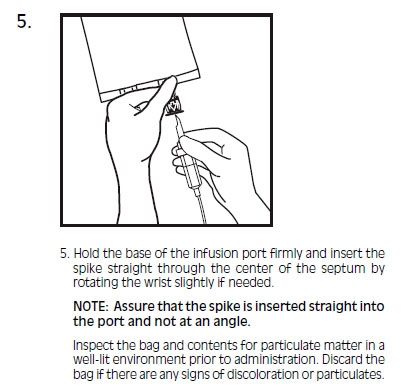
- SPL UNCLASSIFIED SECTION
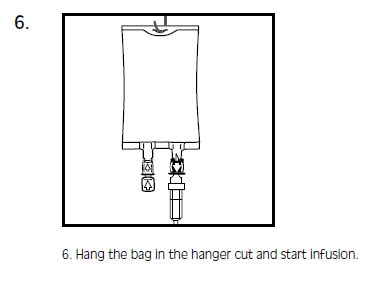
-
DESCRIPTION
Intralipid ® 20% (A 20% Intravenous Fat Emulsion) is a sterile, non-pyrogenic fat emulsion prepared for intravenous administration as a source of calories and essential fatty acids. It is made up of 20% Soybean Oil, 1.2% Egg Yolk Phospholipids, 2.25% Glycerin, and Water for Injection. In addition, sodium hydroxide has been added to adjust the pH so that the final product pH is 8. pH range is 6 to 8.9.
The soybean oil is a refined natural product consisting of a mixture of neutral triglycerides of predominantly unsaturated fatty acids with the following structure:
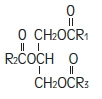
where
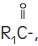
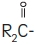 and
and
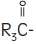 are saturated and unsaturated fatty acid residues.
are saturated and unsaturated fatty acid residues.
The major component fatty acids are linoleic acid (44-62%), oleic acid (19-30%), palmitic acid (7-14%), α-linolenic acid (4-11%) and stearic acid (1.4-5.5%). 1 These fatty acids have the following chemical and structural formulas:
Linoleic acid
C 18H 32O 2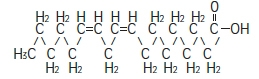
Oleic acid
C 18H 34O 2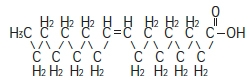
Palmitic acid
C 16H 32O 2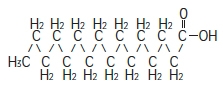
α-Linolenic acid
C 18H 30O 2
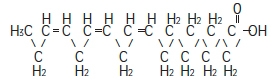
Stearic acid
C 18H 36O 2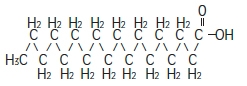
Purified egg phosphatides are a mixture of naturally occurring phospholipids which are isolated from the egg yolk. These phospholipids have the following general structure:
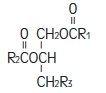
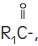 and
and
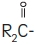 contain saturated and unsaturated fatty acids that abound in neutral fats. R
3 is primarily either the choline or ethanolamine ester of phosphoric acid.
contain saturated and unsaturated fatty acids that abound in neutral fats. R
3 is primarily either the choline or ethanolamine ester of phosphoric acid.
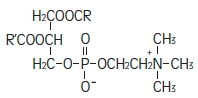
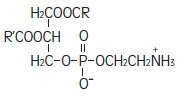
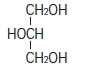
Phosphatidylcholine Phosphatidylethanolamine Glycerin is chemically designated C 3H 8O 3 and is a clear colorless, hygroscopic syrupy liquid. It has the following structural formula:
Intralipid ® 20% (A 20% Intravenous Fat Emulsion) has an osmolality of approximately 350 mOsmol/kg water (which represents 260 mOsmol/L of emulsion) and contains emulsified fat particles of approximately 0.5 micron size.
The total caloric value, including fat, phospholipid and glycerin, is 2.0 kcal per mL of Intralipid ® 20%. The phospholipids present contribute 47 milligrams or approximately 1.5 mmol of phosphorus per 100 mL of the emulsion.
The primary plastic container (Biofine™), is made from multilayered film specifically designed for parenteral nutrition drug products. The film is polypropylene based comprising three co-extruded layers. It contains no plasticizers and exhibits virtually no leachables. The container does not contain DEHP (di(2-ethylhexyl)phthalate) or PVC. The container is nontoxic and biologically inert. This product is not made with natural rubber latex.
The container-emulsion unit is a closed system and is not dependent upon entry of external air during administration.
The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
-
CLINICAL PHARMACOLOGY
Intralipid ® 20% is metabolized and utilized as a source of energy causing an increase in heat production, decrease in respiratory quotient and increase in oxygen consumption. The infused fat particles are cleared from the blood stream in a manner thought to be comparable to the clearing of chylomicrons.
Intralipid ® 20% will prevent the biochemical lesions of essential fatty acid deficiency (EFAD), and correct the clinical manifestations of the EFAD syndrome.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Deaths in preterm infants after infusion of intravenous fat emulsion have been reported in the medical literature. 2 Autopsy findings included intravascular fat accumulation in the lungs. Treatment of premature and low birth weight infants with intravenous fat emulsion must be based upon careful benefit-risk assessment. Strict adherence to the recommended total daily dose is mandatory; hourly infusion rate should be as slow as possible in each case and should not in any case exceed 1 g fat/kg in four hours. Premature and small for gestational age infants have poor clearance of intravenous fat emulsion and increased free fatty acid plasma levels following fat emulsion infusion; therefore, serious consideration must be given to administration of less than the maximum recommended doses in these patients in order to decrease the likelihood of intravenous fat overload. The infant’s ability to eliminate the infused fat from the circulation must be carefully monitored (such as serum triglycerides and/or plasma free fatty acid levels). The lipemia must clear between daily infusions.
Caution should be exercised in administering of Intralipid ® 20% (A 20% Intravenous Fat Emulsion) to patients with severe liver damage, pulmonary disease, anemia or blood coagulation disorders, or when there is danger of fat embolism.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
When Intralipid ® 20% is administered, the patients capacity to eliminate the infused fat from the circulation must be monitored by use of an appropriate laboratory determination of serum triglycerides. Overdosage must be avoided.
During long term intravenous nutrition with Intralipid ® 20%, liver function tests should be performed. If these tests indicate that liver function is impaired, the therapy should be withdrawn.
Frequent (some advise daily) platelet counts should be done in neonatal patients receiving parenteral nutrition with Intralipid ® 20%.
Drug product contains no more than 25 mcg/L of aluminum.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies with Intralipid ® have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy Category C: Animal reproduction studies have not been conducted with Intralipid ®. It is also not known whether Intralipid ® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Intralipid ® should be given to a pregnant woman only if clearly needed.
Nursing Mothers: Caution should be exercised when Intralipid ® is administered to a nursing woman.
Pediatric Use: See DOSAGE AND ADMINISTRATION.
AVOID OVERDOSAGE ABSOLUTELY.
-
ADVERSE REACTIONS
The adverse reactions observed can be separated into two classes:
- Those more frequently encountered are due: either to contamination of the intravenous catheter and result in sepsis, or to vein irritation by concurrently infused hypertonic solutions and may result in thrombophlebitis. These adverse reactions are inseparable from the hyperalimentation procedure with or without Intralipid ® 20% (A 20% I.V. Fat Emulsion).
- Less frequent reactions more directly related to Intralipid ® 20% are: a) immediate or early adverse reactions, each of which has been reported to occur in clinical trials, in an incidence of less than 1%; dyspnea, cyanosis, allergic reactions, hyperlipemia, hypercoagulability, nausea, vomiting, headache, flushing, increase in temperature, sweating, sleepiness, pain in the chest and back, slight pressure over the eyes, dizziness, and irritation at the site of infusion, and, rarely, thrombocytopenia in neonates; b) delayed adverse reactions such as hepatomegaly, jaundice due to central lobular cholestasis, splenomegaly, thrombocytopenia, leukopenia, transient increases in liver function tests, and overloading syndrome (focal seizures, fever, leukocytosis, hepatomegaly, splenomegaly and shock).
The deposition of a brown pigmentation in the reticuloendothelial system, the so-called “intravenous fat pigment,” has been reported in patients infused with Intralipid ® 20%. The causes and significance of this phenomenon are unknown.
-
OVERDOSAGE
In the event of fat overload during therapy, stop the infusion of Intralipid ® 20% until visual inspection of the plasma, determination of triglyceride concentrations, or measurement of plasma light-scattering activity by nephelometry indicates the lipid has cleared. Re-evaluate the patient and institute appropriate corrective measures. See WARNINGS and PRECAUTIONS.
-
DOSAGE AND ADMINISTRATION
Intralipid ® 20% should be administered as a part of Intravenous nutrition via peripheral vein or by central venous infusion.
Adult Patients
The initial rate of infusion in adults should be 0.5 mL/minute for the first 15 to 30 minutes of infusion. If no untoward reactions occur (see ADVERSE REACTIONS section), the infusion rate can be increased to 1 mL/minute. Not more than 500 mL of Intralipid ® 20% should be infused into adults on the first day of therapy. If the patient has no untoward reactions, the dose can be increased on the following day. The daily dosage should not exceed 2.5 g of fat/kg of body weight (12.5 mL of Intralipid ® 20% per kg). Intralipid ® 20% (A 20% I.V. Fat Emulsion) should make up no more than 60% of the total caloric input to the patient. Carbohydrate and a source of amino acids should comprise the remaining caloric input.
Pediatric Patients
The dosage for premature infants starts at 0.5 g fat/kg body weight/24 hours (2.5 mL Intralipid ® 20%) and may be increased in relation to the infant’s ability to eliminate fat. The maximum dosage recommended by the American Academy of Pediatrics is 3 g fat/kg/24 hours. 3
The initial rate of infusion in older pediatric patients should be no more than 0.05 mL/minute for the first 10 to 15 minutes. If no untoward reactions occur, the rate can be changed to permit infusion of 0.5 mL of Intralipid ® 20%/kg/hour. The daily dosage should not exceed 3 g of fat/kg of body weight. 3 Intralipid ® 20% should make up no more than 60% of the total caloric input to the patient. Carbohydrate and a source of amino acids should comprise the remaining caloric input.
Essential Fatty Acid Deficiency
When Intralipid ® 20% is administered to correct essential fatty acid deficiency, eight to ten percent of the caloric input should be supplied by Intralipid ® 20% in order to provide adequate amounts of linoleic and linolenic acids. When EFAD occurs together with stress, the amount of Intralipid ® 20% needed to correct the deficiency may be increased.
Administration
See MIXING GUIDELINES AND LIMITATIONS section for information regarding mixing this fat emulsion with other parenteral fluids.
Intralipid ® 20% can be infused into the same central or peripheral vein as carbohydrate/amino acids solutions by means of a Y-connector near the infusion site. This allows for mixing of the emulsion immediately before entering the vein or for alternation of each parenteral fluid. If infusion pumps are used, flow rates of each parenteral fluid should be controlled with a separate pump. Fat emulsion may also be infused through a separate peripheral site. Use a 1.2 micron filter with Intralipid ® 20%. Filters of less than 1.2 micron pore size must not be used. Conventional administration sets and TPN pooling bags contain polyvinyl chloride (PVC) components that have DEHP (di(2-ethylhexyl) phthalate) as a plasticizer. Fat‑containing fluids such as Intralipid ® 20% extract DEHP from these PVC components and it may be advisable to consider infusion of Intralipid ® 20% through a non-DEHP administration set.
Do not use any bag in which there appears to be an oiling out on the surface of the emulsion.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
MIXING GUIDELINES AND LIMITATIONS
Intralipid ® 20% (A 20% I.V. Fat Emulsion) may be mixed with Amino Acid and Dextrose Injections where compatibility have been demonstrated. Additives known to be incompatible should not be used. Please consult with pharmacist. If, in the informed judgment of the physician, it is deemed advisable to introduce additives, use aseptic technique. Mix thoroughly when additives have been introduced. Do not store solutions containing additives (e.g., Vitamins and Minerals).
When being mixed the following proper mixing sequence must be followed to minimize pH related problems by ensuring that typically acidic Dextrose Injections are not mixed with lipid emulsions alone:
1. Transfer Dextrose Injection to the TPN Admixture Container
2. Transfer Amino Acid Injection
3. Transfer Intralipid ® 20% (A 20% Intravenous Fat Emulsion)
Note: Amino Acid Injection, Dextrose Injection and Intralipid ® 20% may be simultaneously transferred to the admixture container. Admixing should be accompanied by gentle agitation to avoid localized concentration effects.
Additives must not be added directly to Intralipid ® 20% and in no case should Intralipid ® 20% be added to the TPN container first. Bags should be shaken gently after each addition to minimize localized concentration.
If the admixture is not used immediately, the in-use storage time and conditions prior to use are the responsibility of the user and should normally not be longer than 24 hours at 2-8°C.
After removal from storage at 2-8°C, the admixture should be infused within 24 hours.
It is essential that the admixture be prepared using strict aseptic techniques as this nutrient mixture is a good growth medium for microorganisms.
Supplemental electrolytes, trace metals or multivitamins may be required in accordance with the prescription of the attending physician.
The prime destabilizers of emulsions are excessive acidity (low pH) and inappropriate electrolyte content. Careful consideration should be given to additions of divalent cations (Ca ++ and Mg ++) which have been shown to cause emulsion instability. Amino acid solutions exert a buffering effect protecting the emulsion. The admixture should be inspected carefully for “breaking or oiling out“ of the emulsion. “Breaking or oiling out” is described as the separation of the emulsion and can be visibly identified by a yellowish streaking or the accumulation of yellowish droplets in the admixed emulsion. The admixture should also be examined for particulates. The admixture must be discarded if any of the above is observed.
- HOW SUPPLIED
- STORAGE
-
REFERENCES
- Padley FB: “Major Vegetable Fats,” The Lipid Handbook (Gunstone FD, Harwood JL, Padley FB, eds.), Chapman and Hall Ltd., Cambridge, UK (1986), pp. 88-9.
- Levene MI, Wigglesworth JS, Desai R: Pulmonary fat accumulation after Intralipid ® infusion in the preterm infant. Lancet 1980; 2(8199):815-8.
- American Academy of Pediatrics: Use of intravenous fat emulsion in pediatric patients. Pediatrics 1981; 68:5(Nov) 738-43.
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INTRALIPID
i.v. fat emulsion emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0519 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOYBEAN OIL (UNII: 241ATL177A) (SOYBEAN OIL - UNII:241ATL177A) SOYBEAN OIL 20 g in 100 mL Inactive Ingredients Ingredient Name Strength EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0519-58 100 mL in 1 BAG; Type 0: Not a Combination Product 04/01/2004 2 NDC: 0338-0519-09 250 mL in 1 BAG; Type 0: Not a Combination Product 04/01/2004 3 NDC: 0338-0519-13 500 mL in 1 BAG; Type 0: Not a Combination Product 04/01/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018449 04/01/2004 Labeler - Baxter Healthcare Corporation (005083209) Registrant - Fresenius Kabi Deutschland GmbH (506719546) Establishment Name Address ID/FEI Business Operations Fresenius Kabi AB Uppsala 559785113 analysis(0338-0519) , manufacture(0338-0519)
Trademark Results [Intralipid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INTRALIPID 85342969 4403774 Live/Registered |
Kao Kabushiki Kaisha 2011-06-10 |
 INTRALIPID 72168762 0762641 Live/Registered |
Riker Laboratories, Inc. 1963-05-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.