SKIN-AID-MEDICS ANTIBACTERIAL WIPES KIT FRESH, CITRUS, APPLE, CUCUMBER- benzalkonium chloride kit kit
Skin-aid-Medics Antibacterial Wipes Kit Fresh, Citrus, Apple, Cucumber by
Drug Labeling and Warnings
Skin-aid-Medics Antibacterial Wipes Kit Fresh, Citrus, Apple, Cucumber by is a Otc medication manufactured, distributed, or labeled by Kangna (Zhejiang) Medical Supplies Co., Ltd, Global Beauty Care, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor.
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Skin-aid-Medics Antibacterial Wipes Kit Fresh, Citrus, Apple, Cucumber
-
INGREDIENTS AND APPEARANCE
SKIN-AID-MEDICS ANTIBACTERIAL WIPES KIT FRESH, CITRUS, APPLE, CUCUMBER
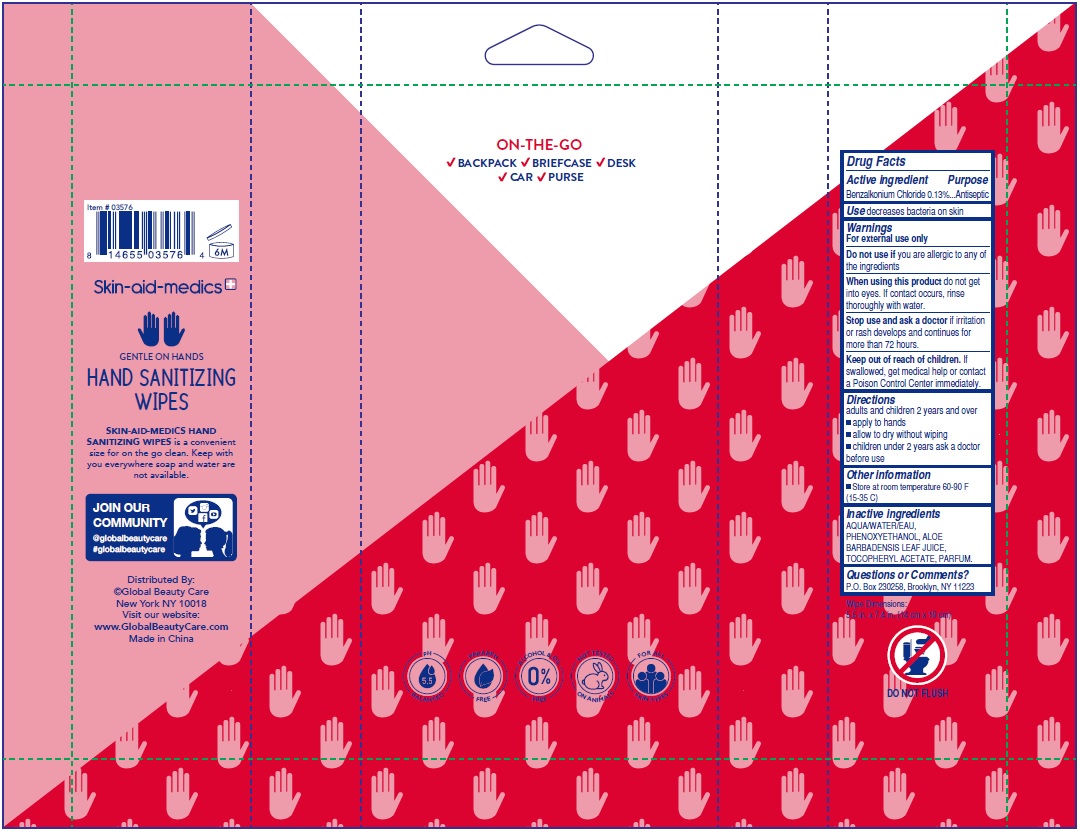
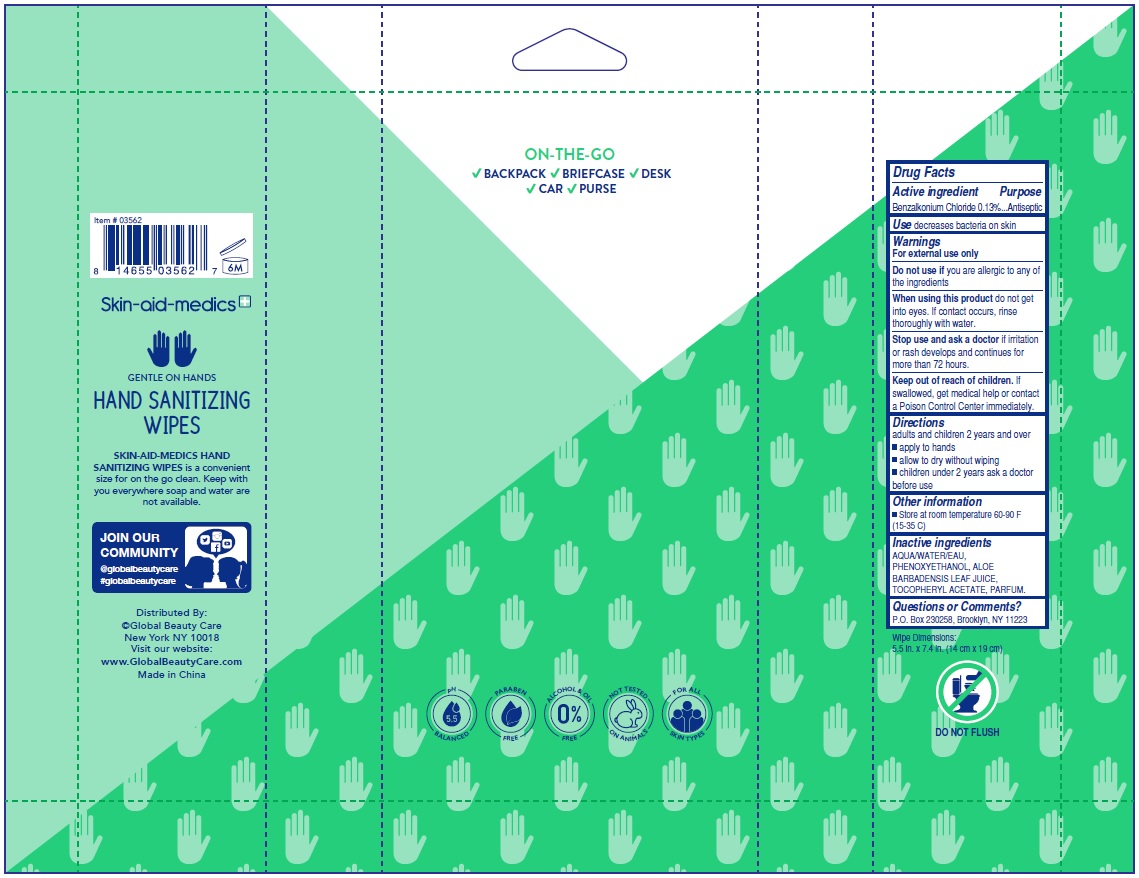
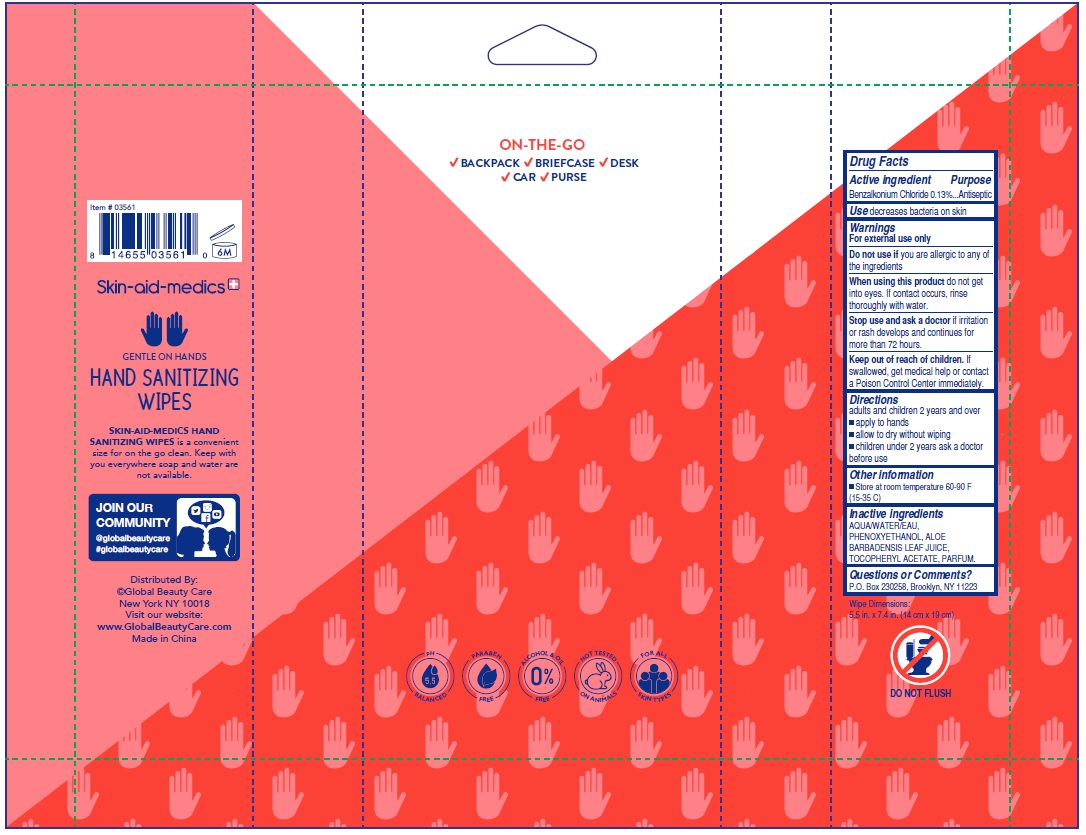
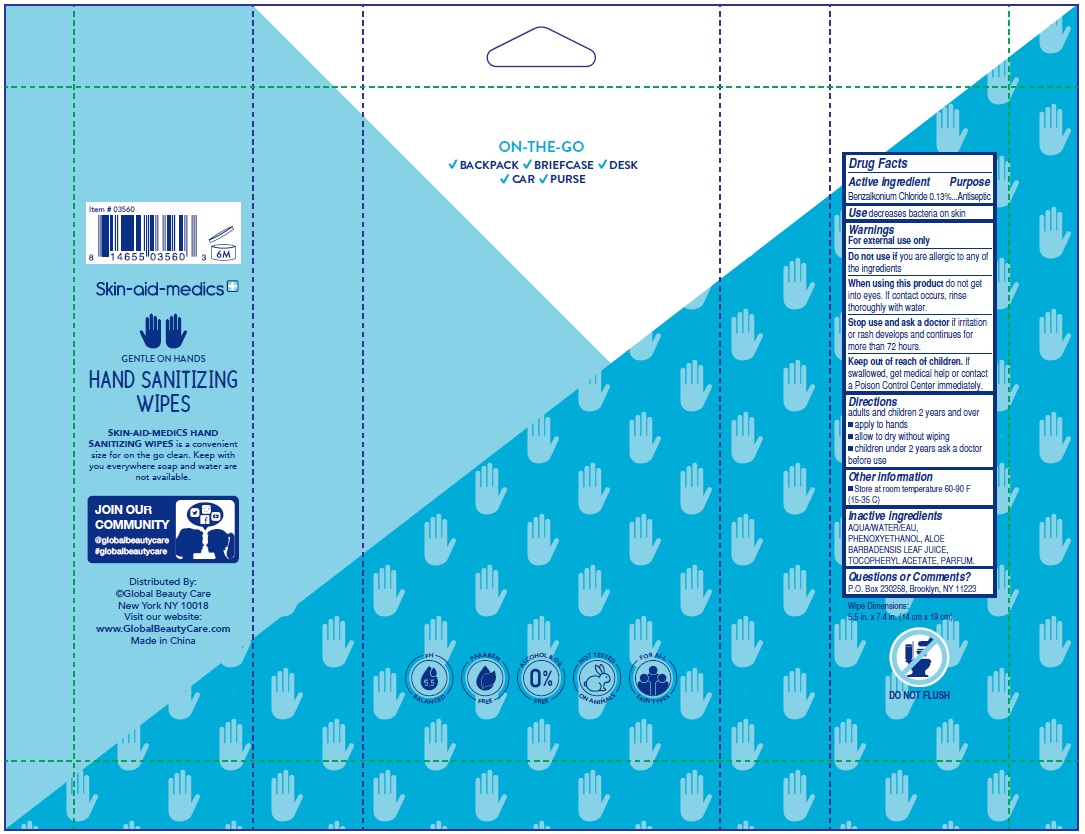
benzalkonium chloride kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 75109-018 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75109-018-40 1 in 1 KIT; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug 05/01/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 POUCH 40 Part 2 1 POUCH 40 Part 3 1 POUCH 40 Part 4 1 POUCH 40 Part 1 of 4 SKIN-AID-MEDICS ANTIBACTERIAL WIPES KIT FRESH SCENT
benzalkonium chloride 0.13% swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75109-007-40 40 in 1 POUCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/01/2020 Part 2 of 4 SKIN-AID-MEDICS ANTIBACTERIAL WIPES KIT CITRUS SCENT
benzalkonium chloride 0.13% swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75109-008-40 40 in 1 POUCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/01/2020 Part 3 of 4 SKIN-AID-MEDICS ANTIBACTERIAL WIPES KIT APPLE SCENT
benzalkonium chloride 0.13% swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75109-011-40 40 in 1 POUCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/01/2020 Part 4 of 4 SKIN-AID-MEDICS ANTIBACTERIAL WIPES KIT CUCUMBER SCENT
benzalkonium chloride 0.13% swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75109-009-40 40 in 1 POUCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/01/2020 Labeler - Kangna (Zhejiang) Medical Supplies Co., Ltd (554530173) Registrant - Global Beauty Care, Inc (068600947)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.