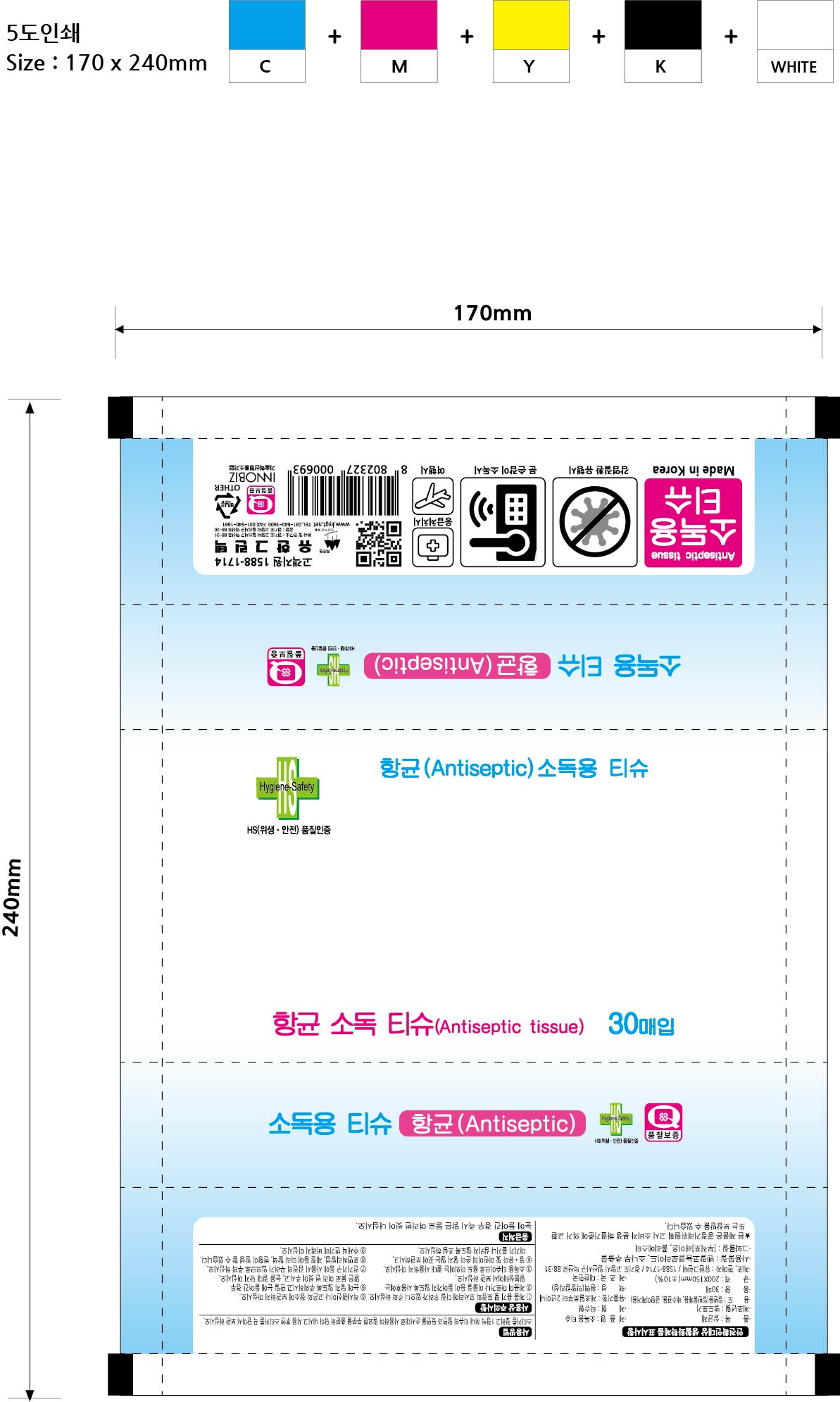
Antiseptic tissue by Yuhan Green Tech Co. sanitizer tissue
Antiseptic tissue by
Drug Labeling and Warnings
Antiseptic tissue by is a Otc medication manufactured, distributed, or labeled by Yuhan Green Tech Co.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTISEPTIC TISSUE- benzalkonium chloride liquid
Yuhan Green Tech Co.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
sanitizer tissue
Direction
Place enough product on hands to cover all surfaces.Rub hands together until dry.
Supervise children under 6 years of age when using this product to avoid swallowing.
When using this product
Keep out of eyes, ears, and mouth in case of contact with eyes, rinse eyes thoroughly whit water.
Keep out of reach of children.
If swallowed get medical help or contact a Poison Control center right away.
| ANTISEPTIC TISSUE
benzalkonium chloride liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Yuhan Green Tech Co. (688304505) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Yuhan Green Tech Co. | 688304505 | manufacture(78583-301) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.