RUBRACA- rucaparib tablet, film coated
Rubraca by
Drug Labeling and Warnings
Rubraca by is a Prescription medication manufactured, distributed, or labeled by Clovis Oncology, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RUBRACA safely and effectively. See full prescribing information for RUBRACA.
RUBRACA® (rucaparib) tablets, for oral use
Initial U.S. Approval: 2016RECENT MAJOR CHANGES
INDICATIONS AND USAGE
RUBRACA is a poly (ADP-ribose) polymerase (PARP) inhibitor indicated:
- for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy. (1.1)
- for the treatment of adult patients with deleterious BRCA mutation (germline and/or somatic)-associated epithelial ovarian, fallopian tube, or primary peritoneal cancer who have been treated with two or more chemotherapies. Select patients for therapy based on an FDA-approved companion diagnostic for RUBRACA. (1.2, 2.3)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 200 mg, 250 mg, and 300 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML): MDS/AML occurred in patients exposed to RUBRACA, and some cases were fatal. Monitor patients for hematological toxicity at baseline and monthly thereafter. Discontinue if MDS/AML is confirmed. (5.1)
- Embryo-Fetal Toxicity: RUBRACA can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.2, 8.1, 8.3)
ADVERSE REACTIONS
- Most common adverse reactions (≥ 20%) were nausea, fatigue (including asthenia), vomiting, anemia, dysgeusia, AST/ALT elevation, constipation, decreased appetite, diarrhea, thrombocytopenia, neutropenia, stomatitis, nasopharyngitis/URI, rash, abdominal pain/distention, and dyspnea (6.1)
- Most common laboratory abnormalities (≥ 25%) were increase in creatinine, increase in ALT, increase in AST, increase in alkaline phosphatase, decrease in hemoglobin, increase in cholesterol, decrease in platelets, decrease in leukocytes, decrease in lymphocytes, and decrease in neutrophils. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Clovis Oncology, Inc. at 1-844-258-7662 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CYP1A2, CYP3A, CYP2C9, and CYP2C19 substrates: Adjust dosage if clinically indicated. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Maintenance Treatment of Recurrent Ovarian Cancer
1.2 Treatment of BRCA-mutated Ovarian Cancer After 2 or More Chemotherapies
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Dose Modifications for Adverse Reactions
2.3 Patient Selection for Treatment of BRCA-mutated Ovarian Cancer
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
5.2 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Rucaparib on Cytochrome p450 (CYP) Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Maintenance Treatment of Recurrent Ovarian Cancer
14.2 Treatment of BRCA-mutated Ovarian Cancer After 2 or More Chemotherapies
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Maintenance Treatment of Recurrent Ovarian Cancer
Rubraca is indicated for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy [see Dosage and Administration (2.1)].
1.2 Treatment of BRCA-mutated Ovarian Cancer After 2 or More Chemotherapies
Rubraca is indicated for the treatment of adult patients with deleterious BRCA mutation (germline and/or somatic)-associated epithelial ovarian, fallopian tube, or primary peritoneal cancer who have been treated with two or more chemotherapies. Select patients for therapy based on an FDA-approved companion diagnostic for Rubraca [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose of Rubraca is 600 mg (two 300 mg tablets) taken orally twice daily with or without food.
Continue treatment until disease progression or unacceptable toxicity.
If a patient misses a dose of Rubraca, instruct the patient to take the next dose at its scheduled time. Vomited doses should not be replaced.
2.2 Dose Modifications for Adverse Reactions
To manage adverse reactions, consider interruption of treatment or dose reduction. Recommended dose reductions are indicated in Table 1.
Table 1. Recommended Dose Adjustments Dose Reduction Dose Starting Dose 600 mg twice daily (two 300 mg tablets) First Dose Reduction 500 mg twice daily (two 250 mg tablets) Second Dose Reduction 400 mg twice daily (two 200 mg tablets) Third Dose Reduction 300 mg twice daily (one 300 mg tablet) 2.3 Patient Selection for Treatment of BRCA-mutated Ovarian Cancer
Select patients for the treatment of epithelial ovarian, fallopian tube, or primary peritoneal cancer with Rubraca based on the presence of a deleterious BRCA mutation (germline and/or somatic) [see Indications and Usage (1.2) and Clinical Studies (14.2)]. Information on the FDA-approved test for the detection of a tumor BRCA mutation in patients with ovarian cancer is available at: http://www.fda.gov/CompanionDiagnostics.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
Myelodysplastic Syndrome (MDS)/Acute Myeloid Leukemia (AML) occur uncommonly in patients treated with Rubraca, and are potentially fatal adverse reactions. In approximately 1100 treated patients, MDS/AML occurred in 12 patients (1.1%), including those in long term follow-up. Of these, 5 occurred during treatment or during the 28 day safety follow-up (0.5%). The duration of Rubraca treatment prior to the diagnosis of MDS/AML ranged from 1 month to approximately 28 months. The cases were typical of secondary MDS/cancer therapy-related AML; in all cases, patients had received previous platinum-containing chemotherapy regimens and/or other DNA damaging agents.
Do not start Rubraca until patients have recovered from hematological toxicity caused by previous chemotherapy (≤ Grade 1). Monitor complete blood counts for cytopenia at baseline and monthly thereafter for clinically significant changes during treatment. For prolonged hematological toxicities (> 4 weeks), interrupt Rubraca or reduce dose according to Table 1 [see Dosage and Administration (2.2)] and monitor blood counts weekly until recovery. If the levels have not recovered to Grade 1 or less after 4 weeks or if MDS/AML is suspected, refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics. If MDS/AML is confirmed, discontinue Rubraca.
5.2 Embryo-Fetal Toxicity
Rubraca can cause fetal harm when administered to a pregnant woman based on its mechanism of action and findings from animal studies. In an animal reproduction study, administration of rucaparib to pregnant rats during the period of organogenesis resulted in embryo-fetal death at exposures that were 0.04 times the AUC0-24h in patients receiving the recommended human dose of 600 mg twice daily. Apprise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of Rubraca [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Myelodysplastic Syndrome/Acute Myeloid Leukemia [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Maintenance Treatment of Recurrent Ovarian Cancer
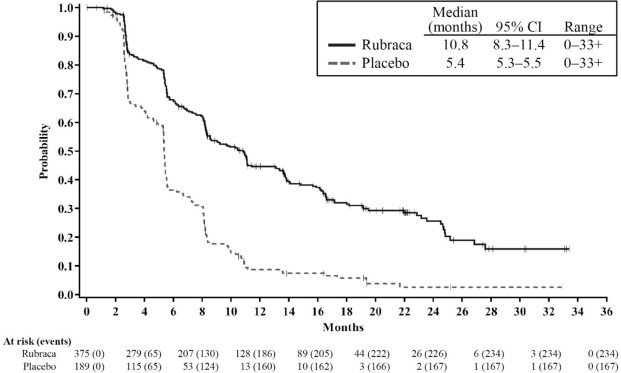
The safety of Rubraca for the maintenance treatment of patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer was investigated in ARIEL3, a randomized (2:1), double-blind, placebo-controlled study in which 561 patients received either Rubraca 600 mg BID (n=372) or placebo (n=189) until disease progression or unacceptable toxicity. The median duration of study treatment was 8.3 months (range: < 1 month to 35 months) for patients who received Rubraca and 5.5 months for patients who received placebo.
Dose interruptions due to an adverse reaction of any grade occurred in 65% of patients receiving Rubraca and 10% of those receiving placebo; dose reductions due to an adverse reaction occurred in 55% of Rubraca patients and 4% of placebo patients. The most frequent adverse reactions leading to dose interruption or dose reduction of Rubraca were thrombocytopenia (18%), anemia (17%), nausea (15%), and fatigue/asthenia (13%). Discontinuation due to adverse reactions occurred in 15% of Rubraca patients and 2% of placebo patients. Specific adverse reactions that most frequently led to discontinuation in patients treated with Rubraca were anemia (3%), thrombocytopenia (3%) and nausea (3%).
Table 2. Adverse Reactions in ARIEL3 Occurring in ≥ 20% of Patients a National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 4.03)
b Consists of grouped related terms that reflect the medical concept of the adverse reaction
Adverse reactions Rubraca
N=372Placebo
N=189Gradesa 1-4
%Grades 3-4
%Gradesa 1-4
%Grades 3-4
%Gastrointestinal Disorders Nausea 76 4 36 0.5 Abdominal pain/distentionb 46 3 39 0.5 Constipation 37 2 24 1 Vomiting 37 4 15 1 Diarrhea 32 0.5 22 1 Stomatitisb 28 1 14 0.5 General Disorders and Administration Site Conditions Fatigue/asthenia 73 7 46 3 Skin and Subcutaneous Tissue Disorders Rashb 43 1 23 0 Nervous System Disorders Dysgeusia 40 0 7 0 Investigations AST/ALT elevation 38 11 4 0 Blood and Lymphatic System Disorders Anemia 39 21 5 0.5 Thrombocytopenia 29 5 3 0 Neutropenia 20 8 5 1 Respiratory, Thoracic, and Mediastinal Disorders Nasopharyngitis/Upper respiratory tract infectionb 29 0.3 18 1 Metabolism and Nutrition Disorders Decreased appetite 23 1 14 0 Adverse reactions occurring < 20% of patients treated with Rubraca include headache (18%), dizziness (19%), dyspepsia (19%), insomnia (15%), dyspnea (17%), pyrexia (13%), peripheral edema (11%), and depression (11%).
Table 3. Laboratory Abnormalities in ARIEL3 Occurring in ≥ 25% of Patients a Patients were allowed to enter clinical studies with laboratory values of CTCAE Grade 1.
Laboratory Parametera Rubraca
N=372Placebo
N=189Grade 1-4
%Grade 3-4
%Grade 1-4
%Grade 3-4
%Chemistry Increase in creatinine 98 0.3 90 0 Increase in cholesterol 84 4 78 0 Increase in ALT 73 7 4 0 Increase in AST 61 1 4 0 Increase in Alkaline Phosphatase 37 0.3 10 0 Hematology Decrease in hemoglobin 88 13 56 1 Decrease in platelets 44 2 9 0 Decrease in leukocytes 44 3 29 0 Decrease in neutrophils 38 6 22 3 Decrease in lymphocytes 29 5 20 3 Treatment of BRCA-mutated Recurrent Ovarian Cancer After 2 or More Chemotherapies
Rubraca 600 mg twice daily as monotherapy has also been studied in 377 patients with epithelial ovarian, fallopian tube or primary peritoneal cancer who have progressed after 2 or more prior chemotherapies in two open-label, single arm trials. In these patients, the median age was 62 years (range: 31 to 86), 100% had an ECOG performance status of 0 or 1, 38% had BRCA-mutated ovarian cancer, 45% had received 3 or more prior lines of chemotherapy, and the median time since ovarian cancer diagnosis was 43 months (range: 6 to 197).
Table 4. Adverse Reactions Reported in ≥ 20% of Patients with Ovarian Cancer After ≥ 2 Chemotherapies Treated with Rubraca in Study 10 and ARIEL2 Adverse Reaction All Ovarian Cancer Patients
(N = 377)
%Gradesa 1-4 Grades 3-4 a National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 4.03)
Gastrointestinal Disorders Nausea 77 5 Vomiting 46 4 Constipation 40 2 Diarrhea 34 2 Abdominal Pain 32 3 General Disorders Asthenia/Fatigue 77 11 Blood and Lymphatic System Disorders Anemia 44 25 Thrombocytopenia 21 5 Nervous System Disorders Dysgeusia 39 0.3 Metabolism and Nutrition Disorders Decreased appetite 39 3 Respiratory, Thoracic, and Mediastinal Disorders Dyspnea 21 0.5 The following adverse reactions have been identified in < 20% of the 377 patients treated with Rubraca 600 mg twice daily: dizziness (17%), neutropenia (15%), rash (includes rash, rash erythematous, rash maculopapular and dermatitis) (13%), pyrexia (11%), photosensitivity reaction (10%), pruritus (includes pruritus and pruritus generalized) (9%), Palmar-plantar erythrodysaesthesia syndrome (2%), and febrile neutropenia (1%).
Table 5. Laboratory Abnormalities Reported in ≥ 35% of Patients with Ovarian Cancer After ≥ 2 Chemotherapies Treated with Rubraca in Study 10 and ARIEL2 a At least one worsening shift in CTCAE grade and by maximum shift from baseline.
b Increase in ALT/AST led to treatment discontinuation in 0.3% of patients (1/377).
Laboratory Parameter All Patients with Ovarian Cancer
(N = 377)
%Grade 1-4 a Grade 3-4 Clinical Chemistry Increase in creatinine 92 1 Increase in ALTb 74 13 Increase in ASTb 73 5 Increase in cholesterol 40 2 Hematologic Decrease in hemoglobin 67 23 Decrease in lymphocytes 45 7 Decrease in platelets 39 6 Decrease in absolute neutrophil count 35 10 -
7 DRUG INTERACTIONS
7.1 Effect of Rucaparib on Cytochrome p450 (CYP) Substrates
Co-administration of rucaparib can increase the systemic exposure of CYP1A2, CYP3A, CYP2C9, or CYP2C19 substrates [see Clinical Pharmacology (12.3)], which may increase the risk of toxicities of these drugs.
Adjust dosage of CYP1A2, CYP3A, CYP2C9, or CYP2C19 substrates, if clinically indicated. If co-administration with warfarin (a CYP2C9 substrate) cannot be avoided, consider increasing the frequency of international normalized ratio (INR) monitoring.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action, Rubraca can cause fetal harm when administered to pregnant women. There are no available data in pregnant women to inform the drug-associated risk. In an animal reproduction study, administration of rucaparib to pregnant rats during organogenesis resulted in embryo-fetal death at maternal exposures that were 0.04 times the AUC0-24h in patients receiving the recommended dose of 600 mg twice daily [see Data]. Apprise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In a dose range-finding embryo-fetal development study, pregnant rats received oral doses of 50, 150, 500, or 1000 mg/kg/day of rucaparib during the period of organogenesis. Post-implantation loss (100% early resorptions) was observed in all animals at doses greater than or equal to 50 mg/kg/day (with maternal systemic exposures approximately 0.04 times the human exposure at the recommended dose based on AUC0-24h).
8.2 Lactation
Risk Summary
There is no information regarding the presence of rucaparib in human milk, or on its effects on milk production or the breast-fed child. Because of the potential for serious adverse reactions in breast-fed children from Rubraca, advise lactating women not to breastfeed during treatment with Rubraca and for 2 weeks following the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiating Rubraca.
Contraception
Females
Rubraca can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of Rubraca.
8.4 Pediatric Use
The safety and effectiveness of Rubraca in pediatric patients have not been established.
8.5 Geriatric Use
In clinical studies 40% (297/749) of patients with ovarian cancer treated with Rubraca were 65 years of age or older and 9% (65/749) were 75 years or older. Grade 3-4 adverse reactions occurred in 65% of patients 65 years or older and in 63% of patients 75 years or older. For patients 65 years or older, the most common Grade 3-4 adverse reactions were anemia, fatigue/asthenia, and ALT/AST increase. No major differences in safety were observed between these patients and younger patients for the maintenance treatment of recurrent ovarian cancer or for the treatment of BRCA-mutated ovarian cancer after two or more chemotherapies.
8.6 Hepatic Impairment
No starting dose adjustment is recommended for patients with mild hepatic impairment (total bilirubin less than or equal to upper limit of normal [ULN] and AST greater than ULN, or total bilirubin between 1.0 to 1.5 times ULN and any AST). No recommendation for starting dose adjustment is available for patients with moderate to severe hepatic impairment (total bilirubin greater than 1.5 times ULN) due to a lack of data [See Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No starting dose adjustment is recommended for patients with mild to moderate renal impairment (baseline creatinine clearance [CLcr] between 30 and 89 mL/min, as estimated by the Cockcroft-Gault method). There is no recommended starting dose for patients with CLcr less than 30 mL/min or patients on dialysis due to a lack of data [See Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
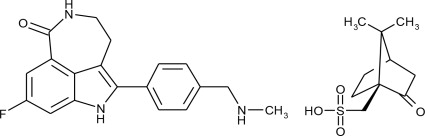
11 DESCRIPTION
Rucaparib is an inhibitor of the mammalian polyadenosine 5'-diphosphoribose polymerase (PARP) enzyme. The chemical name is 8-fluoro-2-{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one ((1S,4R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)methanesulfonic acid salt. The chemical formula of rucaparib camsylate is C19H18FN3OC10H16O4S and the relative molecular mass is 555.67 Daltons.
The chemical structure of rucaparib camsylate is shown below:
Rucaparib camsylate is a white to pale yellow powder; formulated into a tablet for oral use. Rucaparib shows pH-independent low solubility of approximately 1 mg/mL across the physiological pH range.
Rubraca (rucaparib) tablets contain rucaparib camsylate as the active ingredient. Each 200 mg tablet contains 344 mg rucaparib camsylate equivalent to 200 mg rucaparib free base. Each 250 mg tablet contains 430 mg rucaparib camsylate equivalent to 250 mg rucaparib free base. Each 300 mg tablet contains 516 mg rucaparib camsylate equivalent to 300 mg rucaparib free base.
The inactive ingredients in Rubraca tablets include: microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, and magnesium stearate. The cosmetic blue film coating for 200 mg tablets, cosmetic white film coating for 250 mg tablets, and cosmetic yellow film coating for 300 mg tablets is Opadry II containing polyvinyl alcohol, titanium dioxide, polyethylene glycol/macrogol, and talc. The coating is colorized as blue using brilliant blue aluminum lake and indigo carmine aluminum lake, or yellow using yellow iron oxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Rucaparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP-1, PARP-2, and PARP-3, which play a role in DNA repair. In vitro studies have shown that rucaparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, apoptosis, and cancer cell death. Increased rucaparib-induced cytotoxicity and anti-tumor activity was observed in tumor cell lines with deficiencies in BRCA1/2 and other DNA repair genes. Rucaparib has been shown to decrease tumor growth in mouse xenograft models of human cancer with or without deficiencies in BRCA.
12.2 Pharmacodynamics
The pharmacodynamic response of rucaparib has not been characterized.
Cardiac Electrophysiology
The effect of multiple doses of Rubraca on QTc interval was evaluated in an open-label single-arm study in 56 patients with solid tumors who were administered continuous doses of Rubraca ranging from 40 mg once daily (0.03 times the approved recommended dose) to 840 mg twice daily (1.4 times the approved recommended dose). The mean QTcF increase from baseline (90% confidence interval [CI]) in population pharmacokinetics estimated 95th percentile Cmax (3019 ng/mL) at steady state of 600 mg rucaparib twice daily was 14.9 msec (11.1-18.7 msec).
12.3 Pharmacokinetics
The pharmacokinetic profile of rucaparib was characterized in patients with cancer. Rucaparib demonstrated linear pharmacokinetics over a dose range from 240 to 840 mg twice daily with time-independence and dose-proportionality. The mean steady-state rucaparib Cmax was 1940 ng/mL (54% coefficient of variation [CV]) and AUC0-12h was 16900 h·ng/mL (54% CV) at the approved recommended dose. Accumulation was 3.5 to 6.2 fold.
Absorption
The median Tmax was 1.9 hours at the approved recommended dose. The mean absolute bioavailability of rucaparib immediate-release tablet was 36% with a range from 30% to 45%.
Following a high-fat meal, the Cmax was increased by 20% and AUC0-24h was increased by 38%, and Tmax was delayed by 2.5 hours, as compared to dosing under fasted conditions [see Dosage and Administration (2.2)].
Distribution
Rucaparib had a steady-state volume of distribution of 113 L to 262 L following a single intravenous dose of 12 mg to 40 mg rucaparib.
In vitro, the protein binding of rucaparib was 70% in human plasma at therapeutic concentrations. Rucaparib preferentially distributed to red blood cells with a blood-to-plasma concentration ratio of 1.83.
Elimination
The mean terminal T1/2 of rucaparib was 17 to 19 hours, following a single oral dose of 600 mg rucaparib. The apparent clearance ranged from 15.3 to 79.2 L/hour, following rucaparib 600 mg twice daily. The clearance ranged from 13.9 to 18.4 L/hour, following a single intravenous dose of rucaparib 12 mg to 40 mg.
Metabolism
In vitro, rucaparib had a low metabolic turnover rate and was metabolized primarily by CYP2D6 and to a lesser extent by CYP1A2 and CYP3A4.
Specific Populations
Age, Race, and Body Weight
Based on population pharmacokinetic analyses, age, race, and body weight did not have a clinically meaningful effect on rucaparib exposure.
Renal Impairment
In patients who received Rubraca 600 mg twice daily, those with mild renal impairment (N=148; baseline CLcr between 60 and 89 mL/min, as estimated by the Cockcroft-Gault method) and those with moderate renal impairment (N=72; CLcr between 30 and 59 mL/min) showed approximately 15% and 32% higher steady-state AUC, respectively, compared to patients with normal renal function (N=143; CLcr greater than or equal to 90 mL/min). The pharmacokinetic characteristics of rucaparib in patients with CLcr less than 30 mL/min or patients on dialysis are unknown.
Hepatic Impairment
Based on population pharmacokinetic analyses, no apparent pharmacokinetic difference was observed in 34 patients with mild hepatic impairment (total bilirubin less than or equal to ULN and AST greater than ULN, or total bilirubin between 1.0 to 1.5 times ULN and any AST) who received Rubraca 600 mg twice daily as compared to patients with normal hepatic function (N=337). The pharmacokinetic characteristics of rucaparib in patients with moderate to severe hepatic impairment (total bilirubin greater than 1.5 times ULN) are unknown.
Drug Interaction Studies
Effect of Rucaparib on Other Drugs
Clinical Studies
A single dose of the following drugs was administered before and following rucaparib 600 mg twice daily for 7 days. The Cmax of each co-administered drug was ≤ 1.13-fold, and the AUC changed as follows:
- Caffeine (CYP1A2): caffeine AUC increased by 2.55-fold
- Midazolam (CYP3A4): midazolam AUC increased by 1.38-fold
- Warfarin (CYP2C9): warfarin AUC increased by 1.49-fold
- Omeprazole (CYP2C19): omeprazole AUC increased by 1.55-fold
- Digoxin (P-glycoprotein): digoxin AUC increased by 1.20-fold
In Vitro Studies
Rucaparib inhibited CYP2C8, CYP2D6, and uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1). Rucaparib induced CYP1A2, and down regulated CYP3A4 and CYP2B6.
Rucaparib inhibited the P-glycoprotein (P-gp) efflux transporter, breast cancer resistance protein (BCRP), organic anion transporting polypeptides 1B1 and 1B3 (OATP1B1 and OATP1B3), organic anion transporters 1 and 3 (OAT1 and OAT3), multidrug and toxin extrusion 1 and 2-k (MATE1 and MATE2-K), organic cation transporters 1 and 2 (OCT1 and OCT2), and multidrug resistance-associated protein 4 (MRP4). No apparent inhibition was observed for MRP2, MRP3, or BSEP.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with rucaparib.
Rucaparib was clastogenic in an in vitro chromosomal aberration assay in cultured human lymphocytes. The clastogenic response in mitotically-stimulated cells was anticipated based on the mechanism of action of rucaparib and indicates potential genotoxicity in humans. Rucaparib was not mutagenic in a bacterial reverse mutation (Ames) test.
Fertility studies with rucaparib have not been conducted. In 3-month repeat-dose general toxicology studies, rucaparib had no effects on male and female reproductive organs at doses up to 100 mg/kg/day and 20 mg/kg/day in rats and dogs, respectively. These dose levels resulted in systemic exposures of approximately 0.3 and 0.09 times the human exposure (AUC0-24h), respectively, at the recommended dose.
-
14 CLINICAL STUDIES
14.1 Maintenance Treatment of Recurrent Ovarian Cancer
The efficacy of Rubraca was investigated in ARIEL3 (NCT01968213), a double-blind, multicenter clinical trial in which 564 patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who were in response to platinum-based chemotherapy were randomized (2:1) to receive Rubraca tablets 600 mg orally twice daily (n=375) or placebo (n=189). Treatment was continued until disease progression or unacceptable toxicity. All patients had achieved a response (complete or partial) to their most recent platinum-based chemotherapy. Randomization was stratified by best response to last platinum (complete or partial), time to progression following the penultimate platinum therapy (6 to ≤ 12 months and > 12 months), and tumor biomarker status. The major efficacy outcome was investigator-assessed progression-free survival (PFS) evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (v1.1).
The median age was 61 years (range: 39 to 84) for patients receiving Rubraca and 62 years (range: 36 to 85) for those on placebo; the majority were White (80%); and 100% had an ECOG performance status of 0 or 1. All patients had received at least two prior platinum-based chemotherapies (range: 2 to 7). A total of 34% of patients were in complete response (CR) to their most recent therapy. The progression-free interval to penultimate platinum was 6-12 months in 40% of patients and > 12 months in 60%. Prior bevacizumab therapy was reported for 22% of patients who received Rubraca and 23% of patients who received placebo. Measurable disease was present at baseline in 37% of patients.
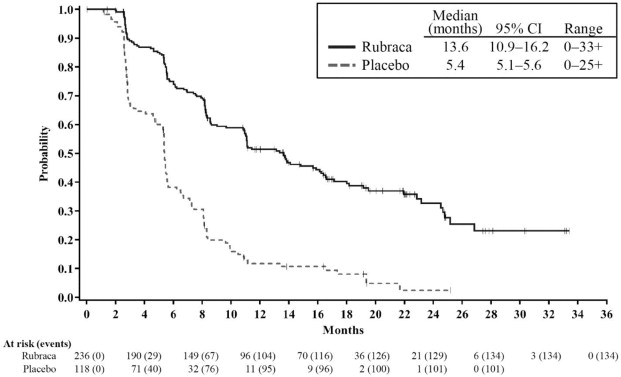
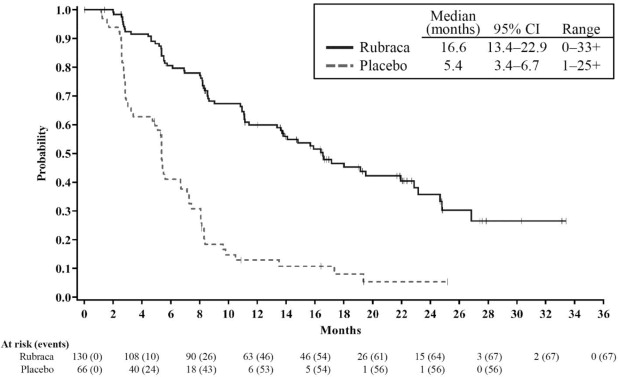
Tumor tissue samples were tested using a clinical trial assay (CTA) (N=564), and the FoundationFocus™ CDx BRCA LOH test (n=518). Of the samples evaluated with both tests, homologous recombination deficiency (HRD) positive status (as defined by the presence of a deleterious BRCA mutation or high genomic loss of heterozygosity) was confirmed by the FoundationFocus™ CDx BRCA LOH test for 94% (313/332) of HRD-positive patients determined by the CTA; and of these, tumor BRCA (tBRCA) mutant status was confirmed by the FoundationFocus™ CDx BRCA LOH test for 99% (177/178) of tBRCA-positive patients determined by the CTA. Blood samples for 94% (186/196) of the tBRCA patients were evaluated using a central blood germline BRCA test. Based on these results, 70% (130/186) of the tBRCA patients had a germline BRCA mutation and 30% (56/186) had a somatic BRCA mutation.
ARIEL3 demonstrated a statistically significant improvement in PFS for patients randomized to Rubraca as compared with placebo in all patients, and in the HRD and tBRCA subgroups. Results from a blinded independent radiology review were consistent. At the time of the analysis of PFS, overall survival (OS) data were not mature (with 22% of events).
Efficacy results are summarized in Table 6 and Figures 1, 2, and 3.
Table 6. Efficacy Results - ARIEL3 (Investigator Assessment) a. All randomized patients.
b. HRD includes all patients with a deleterious germline or somatic BRCA mutation or high genomic loss of heterozygosity, as determined by the CTA.
c. tBRCA includes all patients with a deleterious germline or somatic BRCA mutation, as determined by the CTA.
Rubraca Placebo All Patientsa Patients, N 375 189 PFS events, n (%) 234 (62%) 167 (88%) PFS, median in months 10.8 5.4 HR (95% CI) 0.36 (0.30, 0.45) p-value < 0.0001 HRD Groupb Patients, N 236 118 PFS events, n (%) 134 (57%) 101 (86%) PFS, median in months 13.6 5.4 HR (95% CI) 0.32 (0.24, 0.42) p-value < 0.0001 tBRCA Groupc Patients, N 130 66 PFS events, n (%) 67 (52%) 56 (85%) PFS, median in months 16.6 5.4 HR (95% CI) 0.23 (0.16, 0.34) p-value < 0.0001 Figure 1. Kaplan-Meier Curves of Progression-Free Survival in ARIEL3 as Assessed by Investigator: All Patients
Figure 2. Kaplan-Meier Curves of Progression-Free Survival in ARIEL3 as Assessed by Investigator: HRD Group
Figure 3. Kaplan-Meier Curves of Progression-Free Survival in ARIEL3 as Assessed by Investigator: tBRCA Group
14.2 Treatment of BRCA-mutated Ovarian Cancer After 2 or More Chemotherapies
The efficacy of Rubraca was investigated in 106 patients in two multicenter, single-arm, open-label clinical trials, Study 10 (NCT01482715) and ARIEL2 (NCT01891344), in patients with advanced BRCA-mutant ovarian cancer who had progressed after 2 or more prior chemotherapies. All 106 patients received Rubraca 600 mg orally twice daily as monotherapy until disease progression or unacceptable toxicity. Objective response rate (ORR) and duration of response (DOR) were assessed by the investigator and IRR according to RECIST v1.1.
The median age of the patients was 59 years (range: 33 to 84), the majority were White (78%), and 100% had an ECOG performance status of 0 or 1. All patients had received at least two prior platinum-based chemotherapies and 43% had received 3 or more prior lines of platinum-based chemotherapy. There were 18/106 patients (17%) who had deleterious BRCA mutations detected in tumor tissue and not in whole blood specimens. Tumor BRCA mutation status was verified retrospectively in 96% (64/67) of the patients for whom a tumor tissue sample was available by the companion diagnostic FoundationFocus™ CDxBRCA test, which is FDA approved for selection of patients for Rubraca treatment.
Efficacy results are summarized in Table 7.
Table 7. Overall Response and Duration of Response in Patients with BRCA-mutant Ovarian Cancer Who Received 2 or More Chemotherapies in Study 10 and ARIEL2 Investigator-assessed
N=106Objective Response Rate (95% CI) 54% (44, 64) Complete Response 9% Partial Response 45% Median DOR in months (95% CI) 9.2 (6.6, 11.6) Response assessment by independent radiology review was 42% (95% CI [32, 52]), with a median DOR of 6.7 months (95% CI [5.5, 11.1]). Investigator-assessed ORR was 66% (52/79; 95% CI [54, 76]) in platinum-sensitive patients, 25% (5/20; 95% CI [9, 49]) in platinum-resistant patients, and 0% (0/7; 95% CI [0, 41]) in platinum-refractory patients. ORR was similar for patients with a BRCA1 gene mutation or BRCA2 gene mutation.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Rubraca is available as 200 mg, 250 mg, and 300 mg tablets.
200 mg Tablets:
- Blue, round, and debossed with “C2” on one side
- Supplied in bottles of 60 tablets (NDC: 69660-201-91)
250 mg Tablets:
- White, diamond, and debossed with “C25” on one side
- Supplied in bottles of 60 tablets (NDC: 69660-202-91)
300 mg Tablets:
- Yellow, oval, and debossed with “C3” on one side
- Supplied in bottles of 60 tablets (NDC: 69660-203-91)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
MDS/AML: Advise patients to contact their healthcare provider if they experience weakness, feeling tired, fever, weight loss, frequent infections, bruising, bleeding easily, breathlessness, blood in urine or stool, and/or laboratory findings of low blood cell counts, or a need for blood transfusions. These may be signs of hematological toxicity or a more serious uncommon bone marrow problem called ‘myelodysplastic syndrome’ (MDS) or ‘acute myeloid leukemia’ (AML) which have been reported in patients treated with Rubraca [see Warnings and Precautions (5.1)].
Embryo-Fetal Toxicity: Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment and for 6 months after receiving the last dose of Rubraca [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1, 8.3)].
Photosensitivity: Advise patients to use appropriate sun protection due to the increased susceptibility to sunburn while taking Rubraca [see Adverse Drug Reactions (6.1)].
Lactation: Advise females not to breastfeed during treatment and for 2 weeks after the last dose of Rubraca [see Use in Specific Populations (8.2)].
Dosing Instructions: Instruct patients to take Rubraca orally twice daily with or without food. Doses should be taken approximately 12 hours apart. Advise patients that if a dose of Rubraca is missed or if the patient vomits after taking a dose of Rubraca, patients should not take an extra dose, but take the next dose at the regular time [see Dosage and Administration (2.1)].
Distributed by:
Clovis Oncology, Inc.
Boulder, CO 80301
1-844-258-7662Rubraca is a registered trademark of Clovis Oncology, Inc.
-
PATIENT PACKAGE INSERT
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RUBRACA
rucaparib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69660-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength rucaparib camsylate (UNII: 41AX9SJ8KO) (rucaparib - UNII:8237F3U7EH) rucaparib 200 mg Inactive Ingredients Ingredient Name Strength cellulose, microcrystalline (UNII: OP1R32D61U) sodium starch glycolate type a potato (UNII: 5856J3G2A2) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) Polyvinyl Alcohol, Unspecified (UNII: 532B59J990) titanium dioxide (UNII: 15FIX9V2JP) polyethylene glycol, unspecified (UNII: 3WJQ0SDW1A) talc (UNII: 7SEV7J4R1U) FD&C Blue No. 1 (UNII: H3R47K3TBD) FD&C Blue No. 2 (UNII: L06K8R7DQK) Product Characteristics Color blue (BLUE) Score no score Shape ROUND (ROUND) Size 11mm Flavor Imprint Code C2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69660-201-91 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/19/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209115 12/19/2016 RUBRACA
rucaparib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69660-203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength rucaparib camsylate (UNII: 41AX9SJ8KO) (rucaparib - UNII:8237F3U7EH) rucaparib 300 mg Inactive Ingredients Ingredient Name Strength cellulose, microcrystalline (UNII: OP1R32D61U) sodium starch glycolate type a potato (UNII: 5856J3G2A2) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) Polyvinyl Alcohol, Unspecified (UNII: 532B59J990) titanium dioxide (UNII: 15FIX9V2JP) polyethylene glycol, unspecified (UNII: 3WJQ0SDW1A) talc (UNII: 7SEV7J4R1U) Ferric Oxide Yellow (UNII: EX438O2MRT) Product Characteristics Color yellow (YELLOW) Score no score Shape OVAL (OVAL) Size 16mm Flavor Imprint Code C3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69660-203-91 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/19/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209115 12/19/2016 RUBRACA
rucaparib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69660-202 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength rucaparib camsylate (UNII: 41AX9SJ8KO) (rucaparib - UNII:8237F3U7EH) rucaparib 250 mg Inactive Ingredients Ingredient Name Strength cellulose, microcrystalline (UNII: OP1R32D61U) sodium starch glycolate type a potato (UNII: 5856J3G2A2) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) Polyvinyl Alcohol, Unspecified (UNII: 532B59J990) titanium dioxide (UNII: 15FIX9V2JP) polyethylene glycol, unspecified (UNII: 3WJQ0SDW1A) talc (UNII: 7SEV7J4R1U) Product Characteristics Color white (WHITE) Score no score Shape DIAMOND (DIAMOND) Size 15mm Flavor Imprint Code C25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69660-202-91 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209115 05/01/2017 Labeler - Clovis Oncology, Inc. (830871518)
Trademark Results [Rubraca]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RUBRACA 87273625 5612521 Live/Registered |
Clovis Oncology, Inc. 2016-12-19 |
 RUBRACA 86681510 5156900 Live/Registered |
Clovis Oncology, Inc. 2015-07-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.