CALCITONIN SALMON spray, metered
CALCITONIN SALMON by
Drug Labeling and Warnings
CALCITONIN SALMON by is a Prescription medication manufactured, distributed, or labeled by Par Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CALCITONIN SALMON NASAL SOLUTION (Calcitonin Salmon Nasal Spray) safely and effectively. See full prescribing information for CALCITONIN SALMON NASAL SOLUTION (Calcitonin Salmon Nasal Spray), for Intranasal Use Only (Synthetic Origin).
Initial U.S. Approval: 1975INDICATIONS AND USAGE
Calcitonin Salmon Nasal Solution is a calcitonin, indicated for the treatment of postmenopausal osteoporosis in women greater than 5 years postmenopause when alternative treatments are not suitable. Fracture reduction efficacy has not been demonstrated. (1.1)
Limitations of Use:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Nasal Spray: 2200 International Units per mL of calcitonin-salmon in a 3.7 mL fill glass bottle with screw on pump. Each actuation delivers 200 International Units of calcitonin-salmon (3)
CONTRAINDICATIONS
Hypersensitivity to calcitonin-salmon or any of the excipients (4)
WARNINGS AND PRECAUTIONS
- Serious hypersensitivity reactions including anaphylactic shock have been reported. Consider skin testing prior to treatment in patients with suspected hypersensitivity to calcitonin-salmon. (5.1)
- Hypocalcemia has been reported. Ensure adequate intake of calcium and vitamin D. (5.2)
- Nasal adverse reactions, including severe ulceration can occur. Periodic nasal examinations are recommended. (5.3)
- Malignancy: A meta-analysis of 21 clinical trials suggests an increased risk of overall malignancies in calcitonin-salmon treated patients. (5.4, 6.1)
- Circulating antibodies to calcitonin-salmon may develop, and may cause loss of response to treatment. (5.5)
ADVERSE REACTIONS
Most common adverse reactions (3% or greater) are rhinitis, epistaxis and other nasal symptoms, back pain, arthralgia, and headache (6)
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Concomitant use of calcitonin-salmon and lithium may lead to a reduction in plasma lithium concentrations due to increased urinary clearance of lithium. The dose of lithium may require adjustment (7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and PATIENT COUNSELING INFORMATION.
Revised: 3/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Postmenopausal Osteoporosis
1.2 Important Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Basic Dosing Information
2.2 Priming (Activation) of Pump
2.3 Recommendations for Calcium and Vitamin D Supplementation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Hypocalcemia
5.3 Nasal Adverse Reactions
5.4 Malignancy
5.5 Antibody Formation
5.6 Urine Sediment Abnormalities
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post Marketing Experience
6.3 Immunogenicity
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Treatment of Postmenopausal Osteoporosis
Calcitonin Salmon Nasal Solution is indicated for the treatment of postmenopausal osteoporosis in women greater than 5 years postmenopause. Fracture reduction efficacy has not been demonstrated. Calcitonin Salmon Nasal Solution should be reserved for patients for whom alternative treatments are not suitable (e.g., patients for whom other therapies are contraindicated or for patients who are intolerant or unwilling to use other therapies).
1.2 Important Limitations of Use
- Due to the possible association between malignancy and calcitonin-salmon use, the need for continued therapy should be re-evaluated on a periodic basis [see WARNINGS and PRECAUTIONS (5.4)].
- Calcitonin Salmon Nasal Solution has not been shown to increase spinal bone mineral density in early postmenopausal women.
-
2 DOSAGE AND ADMINISTRATION
2.1 Basic Dosing Information
The recommended dose of Calcitonin Salmon Nasal Solution is 1 spray (200 International Units) per day administered intranasally, alternating nostrils daily.
2.2 Priming (Activation) of Pump
Unopened Calcitonin Salmon Nasal Solution should be stored in the refrigerator. Before using the first dose of Calcitonin Salmon Nasal Solution, the patient should wait until it has reached room temperature. To prime the pump before it is used for the first time, the bottle should be held upright and the two white side arms of the pump depressed toward the bottle, repeat until a full spray is released. The pump is primed once the first full spray is emitted. To administer, the nozzle should first be carefully placed into the nostril while the patient’s head is in the upright position, then the pump should be firmly depressed toward the bottle. The pump should not be primed before each daily dose.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Hypersensitivity to calcitonin-salmon or any of the excipients. Reactions have included anaphylactic shock, anaphylaxis, bronchospasm, and swelling of the tongue or throat [see WARNINGS AND PRECAUTIONS (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions have been reported in patients receiving Calcitonin Salmon Nasal Solution, e.g., bronchospasm, swelling of the tongue or throat, anaphylaxis and anaphylactic shock. Reports of serious hypersensitivity reactions with injectable calcitonin-salmon have also been reported, including reports of death attributed to anaphylaxis. The usual provisions should be made for emergency treatment if such a reaction occurs. Hypersensitivity reactions should be differentiated from generalized flushing and hypotension [see CONTRAINDICATION (4)].
For patients with suspected hypersensitivity to calcitonin-salmon, skin testing should be considered prior to treatment utilizing a dilute, sterile solution of a calcitonin-salmon injectable product. Healthcare providers may wish to refer patients who require skin testing to an allergist. A detailed skin testing protocol is available from the Medical Services Department of Par Pharmaceutical at 1-800-828-9393.
5.2 Hypocalcemia
Hypocalcemia associated with tetany (i.e., muscle cramps, twitching) and seizure activity has been reported with calcitonin therapy. Hypocalcemia must be corrected before initiating therapy with Calcitonin Salmon Nasal Solution. Other disorders affecting mineral metabolism (such as vitamin D deficiency) should also be effectively treated. In patients with these conditions, serum calcium and symptoms of hypocalcemia should be monitored during therapy with Calcitonin Salmon Nasal Solution. Use of Calcitonin Salmon Nasal Solution is recommended in conjunction with an adequate intake of calcium and vitamin D [see DOSAGE AND ADMINISTRATION (2.3)].
5.3 Nasal Adverse Reactions
Adverse reactions related to the nose including rhinitis and epistaxis have been reported. Development of mucosal alterations may occur. Therefore, periodic nasal examinations with visualization of the nasal mucosa, turbinates, septum and mucus, turbinates, septum and mucosal blood vessels are recommended prior to start of treatment with Calcitonin Salmon Nasal Solution, periodically during the course of therapy, at any time nasal symptoms occur.
Calcitonin Salmon Nasal Solution should be discontinued if severe ulceration of the nasal mucosa occurs, as indicated by ulcers greater than 1.5 mm in diameter or penetrating below the mucosa, or those associated with heavy bleeding. Although smaller ulcers often heal without withdrawal of Calcitonin Salmon Nasal Solution, medication should be discontinued temporarily until healing occurs [see ADVERSE REACTIONS (6.1)].
5.4 Malignancy
In a meta-analysis of 21 randomized, controlled clinical trials with calcitonin-salmon (nasal spray or investigational oral formulations), the overall incidence of malignancies reported was higher among calcitonin-salmon treated patients (4.1%) compared with placebo-treated patients (2.9%). This suggests an increased risk of malignancies in calcitonin-salmon treated patients compared to placebo treated patients. The benefits for the individual patient should be carefully considered against possible risks [see ADVERSE REACTIONS (6.1)].
5.5 Antibody Formation
Circulating antibodies to calcitonin-salmon have been reported with Calcitonin Salmon Nasal Solution. The possibility of antibody formation should be considered in any patient with an initial response to Calcitonin Salmon Nasal Solution who later stops responding to treatment [see ADVERSE REACTIONS (6.3)].
5.6 Urine Sediment Abnormalities
Coarse granular casts and casts containing renal tubular epithelial cells were reported in young adult volunteers at bed rest who were given injectable calcitonin-salmon to study the effect of immobilization on osteoporosis. There was no other evidence of renal abnormality and the urine sediment normalized after calcitonin-salmon was stopped. Periodic examinations of urine sediment should be considered. Urine sediment abnormalities have not been reported in ambulatory volunteers treated with calcitonin salmon nasal spray.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Hypersensitivity Reactions, including anaphylaxis [see WARNINGS AND PRECAUTIONS (5.1)].
- Hypocalcemia [see WARNINGS AND PRECAUTIONS (5.2)].
- Nasal Adverse Reactions [see WARNINGS AND PRECAUTIONS (5.3)].
- Malignancy [see WARNINGS AND PRECAUTIONS (5.4)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of Calcitonin Salmon Nasal Solution in the treatment of postmenopausal osteoporosis was assessed in 5 randomized, double-blind, placebo controlled trials that enrolled postmenopausal women, aged 45 to 75 years. The duration of the trials ranged from 1 to 2 years. The incidence of adverse reactions reported in studies involving postmenopausal osteoporotic patients chronically exposed to Calcitonin Salmon Nasal Solution (N=341) and to placebo nasal spray (N=131), and reported in greater than 3% of Calcitonin Salmon Nasal Solution treated patients are presented in the following table. Other than flushing, nausea, possible allergic reactions, and possible local irritative effects in the respiratory tract, a relationship to Calcitonin Salmon Nasal Solution has not been established.
Table 1. Adverse Reactions Occurring in at Least 3% Of Postmenopausal Patients Treated with Calcitonin Salmon Nasal Solution † Symptom of nose includes: nasal crusts, dryness, redness or erythema, nasal sores, irritation, itching, thick feeling, soreness, pallor, infection, stenosis, runny/blocked, small wound, bleeding wound, tenderness, uncomfortable feeling and sore across bridge of nose. Calcitonin Salmon Nasal Solution N = 341
Placebo Nasal Spray N = 131
Adverse Reaction
% of Patients
% of Patients
Rhinitis
12
7
Symptom of Nose†
11
16
Back Pain
5
2
Arthralgia
4
5
Epistaxis
4
5
Headache
3
5
Nasal Adverse Reactions: In all postmenopausal patients treated with Calcitonin Salmon Nasal Solution , the most commonly reported nasal adverse reactions included rhinitis (12%), epistaxis (4%), and sinusitis (2%). Smoking did not have a contributory effect on the occurrence of nasal adverse reactions.
Adverse reactions reported in 1% to 3% of patients treated with Calcitonin Salmon Nasal Solution include: influenza-like symptoms, erythematous rash, arthrosis, myalgia, sinusitis, upper respiratory tract infection, bronchospasm, abdominal pain, nausea, dizziness, paresthesia, abnormal lacrimation, conjunctivitis, lymphadenopathy, infection, and depression.
Malignancy
A meta-analysis of 21 randomized, controlled clinical trials with calcitonin-salmon (nasal spray or investigational oral formulations) was conducted to assess the risk ofmalignancies in calcitonin-salmon treated patients compared to placebo-treatedpatients. The trialsin the meta-analysis ranged in duration from6 months to 5 years and included a total of10883 patients (6151 treated with calcitonin-salmon and 4732 treated with placebo). The overall incidence of malignancies reportedin these 21 trials was higher among calcitonin-salmon- treated patients (254/6151 or 4.1%) compared with placebo-treated patients (137/4732 or 2.9%). Findings were similar when analyses were restricted to the 18 nasal spray only trials [calcitonin-salmon 122/2712 (4.5%); placebo 30/1309 (2.3%)].
The meta-analysis results suggest anincreased risk of overall malignanciesin calcitonin-salmon-treated patients compared to placebo-treated patientswhen all 21trials are included and when the analysis is restricted to the 18 nasal spray only trials (see Table 2). It is not possible to exclude an increased risk when calcitonin-salmon is administered by the subcutaneous, intramuscular, or intravenous route because these routes of administration were not investigated in the meta-analysis. The increased malignancy risk seenwith the meta-analysis was heavily influenced by a single large 5-year trial, which had an observed riskdifference of 3.4% [95% CI (0.4%, 6.5%)]. Imbalances in risks were still observed when analysesexcluded basal cell carcinoma (see Table 2); the data were not sufficient for further analyses by type of malignancy. A mechanism for these observations has not been identified. Although a definitive causal relationship between calcitonin-salmon use and malignancies cannot be established from this meta-analysis, the benefits for the individual patient should be carefully evaluated against all possible risks [see WARNINGS AND PRECAUTIONS (5.4)].
Table 2: Risk Difference for Malignancies in Calcitonin-Salmon Treated Patients Compared with Placebo-Treated Patients
Patients
Malignancies
Risk Difference 1 (%)
95% Confidence Interval 2 (%)
All (nasal spray + oral)
All
1.0
(0.3, 1.6)
All (nasal spray + oral)
Excluding basal cell carcinoma
0.5
(-0.1, 1.2)
All (nasal spray only)
All
1.4
(0.3, 2.6)
All (nasal spray only)
Excluding basal cell carcinoma
0.8
(-0.2, 1.8)
1 The overall adjusted risk difference is the difference between the percentage of patients who had any malignancy (or malignancy excluding basal cell carcinoma) in calcitonin-salmon and placebo treatment groups, using the Mantel-Haenszel (MH) fixed-effect method. A risk difference of 0 is suggestive of no difference in malignancy risks between the treatment groups.
2 The corresponding 95% confidence interval for the overall adjusted risk difference also based on MH fixed-effect method.
6.2 Post Marketing Experience
Because postmarketing adversereactions are reported voluntarily froma population of uncertain size, it is not always possible to reliably estimate their frequencyor establish a causal relationship to drug exposure.
The following adverse reactions have been reported during post-approval use of Calcitonin Salmon Nasal Solution.
- Allergic/Hypersensitivity Reactions:Serious allergic reactions have been reported in patients receiving Calcitonin Salmon Nasal Solution, including anaphylaxis and anaphylactic shock.
- Hypocalcemia:Hypocalcemia with paresthesia has been reported.
- Body as a Whole: facial or peripheral edema
- Cardiovascular: hypertension, vasodilatation, syncope, chest pain
- Nervous system: dizziness, seizure, visual or hearing impairment, tinnitus
- Respiratory/Special Senses: cough, bronchospasm, dyspnea, loss of taste/smell
- Skin: rash/dermatitis, pruritus, alopecia, increased sweating
- Gastrointestinal: diarrhea
- Nervous System Disorders: tremor
6.3 Immunogenicity
Consistent with the potentiallyimmunogenic properties of medicinal products containing peptides, administration of Calcitonin Salmon Nasal Solution may trigger the development ofanti-calcitonin antibodies.In a two-year Calcitonin Salmon Nasal Solution clinicalstudy that evaluated immunogenicity, a measurable antibodytiter was found in 69% of patients treated with Calcitonin Salmon Nasal Solution and 3% of placebo-treated patients. Antibody formation may be associated with a loss of response to treatment [see WARNINGS AND PRECAUTIONS (5.5)].
The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of a positive antibodytest result may be influenced by several factors, including assay methodology, sample handling, timing ofsample collection, concomitant medications, and underlying disease. For these reasons, comparison of antibodies to Calcitonin Salmon Nasal Solution with the incidence of antibodies to other calcitonin-containing products may be misleading.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Calcitonin Salmon Nasal Solution is not indicated for use in females of reproductive potential. There are no data with the use of Calcitonin Salmon Nasal Solution in pregnant women. In an animal reproduction study, subcutaneous administration of calcitonin-salmon to pregnant rabbits during organogenesis at 4 to 18 times the recommended parenteral human dose caused a decrease in fetal birth weights. No adverse developmental outcome was observed in the rat with subcutaneous administration of calcitonin-salmon at 9 times the recommended human parenteral dose based on body surface area (see Data).
Data
Animal Data
Calcitonin-salmon has been shown to cause a decrease in fetal birth weights in rabbits when given by subcutaneous injection in doses 4 to 18 times the parenteral dose (of 54 International Units/m2) and 70 to 278 times the intranasal dose recommended for human use based on body surface area. No embryo/fetal toxicities related to Calcitonin Salmon Nasal Solution were reported from maternal subcutaneous daily doses in rats up to 80 International Units/kg/day from gestation day 6 to 15.
8.2 Lactation
Risk Summary
Calcitonin Salmon Nasal Solution is not indicated for use in females of reproductive potential. There is no information on the presence of calcitonin-salmon in human milk, the effects on the breastfed child, or the effects on milk production. Calcitonin has been shown to inhibit lactation in rats.
8.5 Geriatric Use
In a multi-centered, double-blind, randomized clinical study of calcitonin salmon nasal spray, 279 patients were less than 65 years old, while 467 patients were 65 to 74 years old and 196 patients were 75 years old and older. Compared to subjects less than 65 years old, the incidence of nasal adverse reactions (rhinitis, irritation, erythema, and excoriation) was higher in patients over the age of 65, particularly among those over the age of 75. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
10 OVERDOSAGE
The pharmacologic actions of Calcitonin Salmon Nasal Solutionsuggest that hypocalcemictetany could occur in overdose. Therefore, provisions for parenteral administration of calcium should be available for the treatment of overdose.
Single doses of Calcitonin Salmon Nasal Solution up to 1600 International Units, doses up to 800 International Units per day for 3 days and chronic administration of doses up to 600 International Units per day have been studied without serious adverse effects.
-
11 DESCRIPTION
Calcitonin is a polypeptide hormone secreted by the parafollicular cells of the thyroid gland in mammals and by the ultimobranchial gland of birds and fish.
Calcitonin Salmon Nasal Solution is a synthetic polypeptide of 32 amino acids in the same linear sequence that is found in calcitonin of salmon origin. This is shown by the following graphic formula:
It is provided in a 3.7 mL fill glass bottle as a solution for nasal administration. This is sufficient medication for at least 30 doses.
Active Ingredient: calcitonin-salmon, 2200 International Units per mL (corresponding to 200 International Units per 0.09 mL actuation).
Inactive Ingredients: sodium chloride, chlorobutanol (possesses a characteristic odor), hydrochloric acid (added as necessary to adjust pH), purified water and nitrogen.
The activity of Calcitonin Salmon Nasal Solution is stated in International Units based on bioassay in comparison with the International Reference Preparation of calcitonin-salmon for Bioassay, distributed by the National Institute of Biologic Standards and Control, Holly Hill, London.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Calcitonin-salmon is a calcitonin receptor agonist. Calcitonin-salmon acts primarily on bone, but direct renal effects and actions on the gastrointestinal tract are also recognized. Calcitonin-salmon appears to have actions essentially identical to calcitonins of mammalian origin, but its potency per mg is greater and it has a longer duration of action.
The actions of calcitonin on bone and its role in normal human bone physiology are still not completely elucidated, although calcitonin receptors have beendiscoveredin osteoclasts and osteoblasts.
12.2 Pharmacodynamics
The information below, describing the clinical pharmacology of calcitonin, has been derived from studies with injectable calcitonin-salmon. The mean bioavailability of calcitonin-salmon nasal spray is approximately 3% of the injectable calcitonin-salmon in healthy subjects and, therefore, theconclusionsconcerningtheclinical pharmacology of this preparation may be different.
Bone
Single injections of calcitonin-salmon caused a marked transient inhibition of the ongoing bone resorptive process. With prolonged use, there is a persistent, smaller decrease in the rate of bone resorption. Histologically, this is associated witha decreasednumber of osteoclasts and an apparent decrease in their resorptive activity.
In healthy adults, who have a relatively low rate of bone resorption, theadministration of exogenous calcitonin‑ salmon results in decreases in serumcalciumwithin thelimits of the normal range. In healthy children and in patients whose bone resorption is more rapid, decreasesin serumcalciumare more pronounced in response to calcitonin-salmon.
Kidney
Studies withinjectable calcitonin-salmon show increasesin the excretionof filtered phosphate, calcium, and sodiumby decreasing their tubular reabsorption. Comparable studieshave not been conducted with Calcitonin Salmon Nasal Solution.
Gastrointestinal Tract
Some evidence fromstudies with injectable preparations suggests that calcitonin-salmon may have effects on the gastrointestinal tract.Short-termadministration of injectable calcitonin-salmon results in marked transient decreases in the volume and acidity ofgastric juice and in the volume andthe trypsin and amylase content of pancreatic juice. Whether these effects continue to beelicited after each injection of calcitonin-salmon during chronic therapy has not been investigated. These studies have not beenconducted with Calcitonin Salmon Nasal Solution.
CalciumHomeostasis
In two clinical studies designed toevaluate the pharmacodynamic response to calcitonin-salmon nasal spray, administration of calcitonin-salmon 100 to 1600 International Unitsto healthy volunteers resulted in rapid and sustained decreases within the normal range for both total serumcalciumand serumionized calcium. Single doses of calcitonin-salmon greater than 400 International Units did notproduce any further biological response to the drug.
12.3 Pharmacokinetics
The bioavailability of Calcitonin Salmon Nasal Solution relative to intramuscular administration in healthy volunteers is between 3 and 5%. Calcitonin Salmon Nasal Solution is absorbed rapidly by the nasal mucosa with a mean Tmax of about 13 minutes. The terminal half-life of calcitonin-salmon has been calculated to be around 18 minutes and no evidence of accumulation was observed with multiple dosing.
.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
The incidence of pituitary adenomas was increased in rats after one and two years of subcutaneous exposure to synthetic calcitonin-salmon. The significance of this finding to humansis unknown because pituitary adenomas are very common in rats as they age, the pituitary adenomas did not transforminto metastatic tumors, there were no other clear treatment-related neoplasms, and synthetic calcitonin salmon related neoplasms were not observed in mice after two years of dosing.
Rat findings:
The only clear neoplastic finding in rats dosed subcutaneously with synthetic calcitonin-salmon was an increase in the incidence of pituitary adenomas in male Fisher 344rats and female Sprague Dawley rats after one year of dosing and male Sprague Dawley rats dosed for one and two years. In female Sprague Dawley rats, the incidence of pituitary adenomas after two years was high in all treatment groups (between 80% and 92% including the control groups) such that a treatment-related effectcould not be distinguished fromnatural backgroundincidence.Thelowestdosein male Sprague Dawley rats thatdeveloped an increased incidence of pituitary adenomas after two yearsof dosing (1.7 International Units/kg/day) is approximately 2 times the maximum recommended intranasal dose in humans (200International Units/day) based on body surface area conversion between rats and humansand a 20-fold conversion factor to account for decreased clinical exposure via the intranasal route. The findingssuggest that calcitonin-salmon reducedthe latency period for development of non-functioning pituitary adenomas.
Mouse findings:
No carcinogenicity potential was evident in male or female mice dosed subcutaneously for two years with synthetic calcitonin-salmon at dosesup to 800 International Units/kg/day. The 800 International Units/kg/day dose is approximately 390 times the maximum recommended intranasal dose in humans (200 International Units) basedon scaling for body surface area anda 20-foldconversion factor to account for low clinical exposure via the intranasal route.
Mutagenesis
Synthetic calcitonin-salmon testednegative for mutagenicity usingSalmonella typhimurium(5strains) and Escherichiacoli (2 strains), with and withoutrat liver metabolic activation, and was not clastogenic in a chromosome aberration test in Chinese Hamster V79cells. There was no evidence that calcitonin- salmon was clastogenic in the in vivo mouse micronucleustest.
Fertility
Effects ofcalcitonin-salmon on fertility have not been assessed in animals.
-
14 CLINICAL STUDIES
Two randomized, placebo-controlled, two-year trials were conducted in 266 postmenopausal women who were greater than 5 years postmenopause with spinal, forearmor femoral bone mineral density (BMD) at least one standard deviation belowthe normal value for healthy premenopausal women (T-score < -1). In both studies, a total of 144 patients receivedCalcitonin Salmon Nasal Solution 200 International Units or placebodaily. The intent-to-treat population comprised 139 patients who had at least one follow-up BMD measurement. In study 1, patients also received 500 mg daily calciumsupplements, while in study 2, patients received no calciumsupplementation. The primary endpoint for both studieswas percent change in lumbar spine BMD at 2 years. Calcitonin Salmon Nasal Solution increased lumbar vertebral BMD relative to placebo in women with low bone mass who were greater than 5 years post menopause (see Table 3 below).
Table 3. Calcitonin Salmon Nasal Solution: Lumbar Spine Bone Mineral Density in Women Greater Than 5 years Postmenopause With Low Bone Mass
Lumber Spine Bone Mineral Density, Mean Change From Baseline (in %) at Month 24
Study 1 (with calcium supplement) n (ITT) = 100
Study 2 (no calcium supplement) n(ITT) = 39
Calcitonin Salmon Nasal Solution 200 IU NS daily
+1.56
+1.02
Placebo
+0.20
-1.85
Treatment Difference
+1.36
+2.87
p-value†
<0.05
<0.005
ITT: Intent To Treat
IU: International Units
NS: nasal spray
p – values by parametric testing (2 – tailed 2-sample t-test)
No effects of calcitonin salmon nasal spray on cortical bone of the forearm or hip were demonstrated.
In clinical studies of postmenopausal osteoporosis, bone biopsy and radial bone mass assessments at baseline and after 26 months of daily injectable calcitonin-salmon indicate that calcitonin therapy results in the formation of normal bone.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
HOW SUPPLIED
Available as a metered dose clear solution in a 3.7 mL fill clear glass bottle. It is available in a dosage strength of 200 International Units per activation (0.09 mL/spray). A screw-on pump is provided. The pump, following priming, will deliver 0.09 mL of solution. Calcitonin Salmon Nasal Solution contains 2200 International Units/mL calcitonin-salmon and is provided in an individual box containing one glass bottle and one screw-on pump (NDC: 49884-161-11).
Storage and Handling
Store unopened bottle in refrigerator between 2°C to 8°C (36°F to 46°F). Freezing is to be avoided.
Store bottle in use at room temperature between 20°C to 25°C (68°F to 77°F) in an upright position, for up to 35 days.
Each bottle contains at least 30 doses. Discard bottle after 30 doses.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use).
- Instruct patients on pump assembly, priming of the pump, and nasal introduction of Calcitonin Salmon Nasal Solution. Although instructions for patients are supplied with the individual bottle, procedures for use should be demonstrated to each patient [see DOSAGE AND ADMINISTRATION (2.2)]. Patients should notify their healthcare provider if they develop significant nasal irritation [see WARNINGS AND PRECAUTIONS (5.3)].
- Inform patients of the potential increase in risk of malignancy [see WARNINGS AND PRECAUTIONS (5.4)].
- Advise patients to maintain an adequate calcium (at least 1000 mg elemental calcium per day) and vitamin D (at least 400 International Units per day) intake [see DOSAGE AND ADMINISTRATION (2.3)].
- Instruct patients to seek emergency medical help or go to the nearest hospital emergency room right away if they develop any signs or symptoms of a serious allergic reaction.
- Advise patients how to correctly store unopened and opened product [see HOW SUPPLIED/STORAGE AND HANDLING (16)]. Advise patients that the bottle should be discarded after 30 doses, because after 30 doses, each spray may not deliver the correct amount of medication even if the bottle is not completely empty.
-
INFORMATION FOR THE PATIENT
Read this Patient Information before you start using Calcitonin Salmon Nasal Solution and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is Calcitonin Salmon Nasal Solution?
Calcitonin Salmon Nasal Solution is a prescription medicine used to treat osteoporosis in women more than 5 years after menopause. Calcitonin Salmon Nasal Solution should be used for women who cannot use other treatments or who choose not to use other treatments for osteoporosis.
It is not known if Calcitonin Salmon Nasal Solution lowers the chance of having bone fractures.
Calcitonin Salmon Nasal Solution has not been shown to be effective in women less than 5 years after menopause.
It is not known if Calcitonin Salmon Nasal Solution is safe and effective in children under 18 years of age.
Who should not use Calcitonin Salmon Nasal Solution?
Do not use Calcitonin Salmon Nasal Solution if you:
- are allergic to calcitonin-salmon or any of the ingredients in Calcitonin Salmon Nasal Solution. See the end of this leaflet for a complete list of ingredients in Calcitonin Salmon Nasal Solution.
What should I tell my healthcare provider before using Calcitonin Salmon Nasal Solution? Before you use Calcitonin Salmon Nasal Solution, tell your healthcare provider if you:
- have any other medical conditions
- have low calcium levels in your blood
- are pregnant or plan to become pregnant. It is not known if Calcitonin Salmon Nasal Solution can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Calcitonin Salmon Nasal Solution passes into your breast milk. You and your healthcare provider should decide if you will use Calcitonin Salmon Nasal Solution or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- lithium. Your healthcare provider may need to change your dose of lithium while you use Calcitonin Salmon Nasal Solution.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Calcitonin Salmon Nasal Solution?
- For detailed instructions, see the Instructions for Use at the end of this Patient Information leaflet.
- Use Calcitonin Salmon Nasal Solution exactly as your healthcare provider tells you to use it.
- Do not use Calcitonin Salmon Nasal Solution until your healthcare provider shows you and you understand how to use it correctly.
- Use 1 spray of Calcitonin Salmon Nasal Solution, 1 time each day, in 1 nostril (inside your nose).
- Start with 1 spray in your left nostril on your first day, followed by 1 spray in your right nostril on the second day.
- Continue to switch nostrils for your dose each day.
- Your healthcare provider should check your nose before you start using Calcitonin Salmon Nasal Solution and often while you are using it.
- Tell your healthcare provider if you start to have discomfort (irritation) in your nose that bothers you while you use Calcitonin Salmon Nasal Solution.
- Your healthcare provider should prescribe calcium and vitamin D to help prevent low calcium levels in your blood while you use Calcitonin Salmon Nasal Solution.
- Take your calcium and vitamin D as your healthcare provider tells you to.
- There are 30 doses (sprays) of Calcitonin Salmon Nasal Solution in each bottle. After 30 doses, each spray may not give you the right amount of medicine, even if the bottle is not completely empty. Keep track of the number of doses of medicine used from your bottle.
- If you use too much Calcitonin Salmon Nasal Solution, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Calcitonin Salmon Nasal Solution?
Calcitonin Salmon Nasal Solution may cause serious side effects, including:
- allergic reactions
Some people have had an allergic reaction when using Calcitonin Salmon Nasal Solution. Some reactions may be serious and can be life threatening. Call your healthcare provider or go to the nearest hospital emergency room right away if you have any of these symptoms of an allergic reaction.
- trouble breathing
- swelling of your face, throat or tongue
- fast heartbeat
- chest pain
- feel dizzy or faint
If you might be allergic to calcitonin-salmon, your healthcare provider should do a skin test before you use Calcitonin Salmon Nasal Solution.
- low calcium levels in your blood (hypocalcemia)
Calcitonin Salmon Nasal Solution may lower the calcium levels in your blood. If you have low blood calcium before you start using Calcitonin Salmon Nasal Solution, it may get worse during treatment. Your low blood calcium must be treated before you use Calcitonin Salmon Nasal Solution. Most people with low blood calcium levels do not have symptoms, but some people may have symptoms. Call your healthcare provider right away if you have any of these symptoms of low blood calcium:
- numbness or tingling in your fingers, toes, or around your mouth
Your healthcare provider should:
- do blood tests while you use Calcitonin Salmon Nasal Solution
- prescribe calcium and vitamin D to help prevent low calcium levels in your blood while you use Calcitonin Salmon Nasal Solution
Take your calcium and vitamin D as your healthcare provider tells you to.
- nose irritation
Irritation of your nose can happen while you are using Calcitonin Salmon Nasal Solution, especially if you are over 65 years of age. Call your healthcare provider right way if you have any of these symptoms of nose irritation
- crusting
- dryness
- redness or swelling
- nose sores (ulcers)
- nose bleeds
Your healthcare provider may stop your treatment with Calcitonin Salmon Nasal Solution until your nose irritation symptoms go away.
- risk of cancer
People who use calcitonin-salmon, the medicine in Calcitonin Salmon Nasal Solution, may have an increased risk of cancer.
- increase of certain cells (sediment) in your urine
Your healthcare provider should test your urine often while you are using Calcitonin Salmon Nasal Solution.
The most common side effects of Calcitonin Salmon Nasal Solution include:
- back pain
- muscle aches
- headache
- runny nose
These are not all the possible side effects of Calcitonin Salmon Nasal Solution. For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider right away if you have any side effect that bothers you or does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800-FDA-1088.
How do I store Calcitonin Salmon Nasal Solution?
- Store open bottles of Calcitonin Salmon Nasal Solution at room temperature between 20°C to 25°C (68°F to 77°F) for 35 days.
- Store unopened bottles of Calcitonin Salmon Nasal Solution in the refrigerator between 2°C to 8°C (36°F to 46°F). Do not freeze.
- Store Calcitonin Salmon Nasal Solution bottles in an upright position.
- Safely throw away Calcitonin Salmon Nasal Solution in the trash after you have used 30 doses (sprays).
Keep Calcitonin Salmon Nasal Solution and all other medicines out of the reach of children.
General information about the safe and effective use of Calcitonin Salmon Nasal Solution.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Calcitonin Salmon Nasal Solution for a condition for which it was not prescribed. Do not give Calcitonin Salmon Nasal Solution to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information summarizes the most important information about Calcitonin Salmon Nasal Solution. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Calcitonin Salmon Nasal Solution that is written for health professionals.
For more information, call 1-800-828-9393.
What are the ingredients in Calcitonin Salmon Nasal Solution?
Active Ingredients: calcitonin-salmon
Inactive Ingredients: sodium chloride, chlorobutanol (possesses a characteristic odor), hydrochloric acid (added as necessary to adjust pH), purified water and nitrogen.
Instructions for Use
Calcitonin (cal·ci·to·nin) SalmonNasal Solution, USP
(Calcitonin Salmon Nasal Spray)
For Nasal Use Only.
Important information about your Calcitonin Salmon Nasal Solution:
-
A single spray of Calcitonin Salmon Nasal Solution contains 1 daily dose of medicine.
-
Each Calcitonin Salmon Nasal Solution bottle contains the right amount of medicine. The bottle may not be completely filled to the top. This is normal.
-
This package contains 1 bottle of Calcitonin Salmon Nasal Solution and 1-screw-on pump.
-
Store unopened bottles of Calcitonin Salmon Nasal Solution in the refrigerator between 36°F to 46°F (2°C to 8°C). Do not freeze.
-
After you open your bottle of Calcitonin Salmon Nasal Solution, store it at room temperature between 20°C to 25°C (68°F to 77°F) in an upright position. Do not shake the bottle.
Preparing your Calcitonin Salmon Nasal Solution:
Step 1. Remove the bottle from your refrigerator and let it reach room temperature. Check the medicine in the bottle to make sure it is clear and colorless without particles.
Important: If your Calcitonin Salmon Nasal Solution bottle and pump have already been put together by your pharmacist or healthcare provider, go to Step 6.
Step 2. Keep the bottle upright and unscrew the white cap. Step 3. Remove the pump from the plastic protection bag. 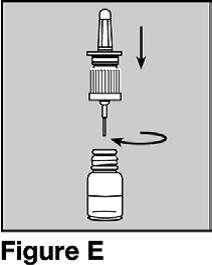
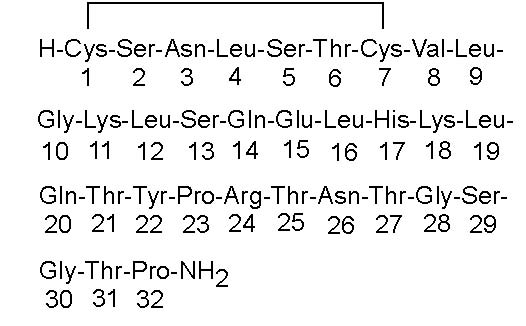
Step 4. Hold the bottle upright and insert the nose spray pump into the bottle. Turn the pump clockwise to tighten it until it is securely attached to the bottle. See Figure E.
Do not push down on the pump when it is not attached to the bottle.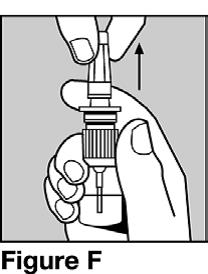
Step 5. Holding the bottle upright with your index finger on top of one of the two side arms of the pump, gently remove the clear protective cap from the top of the nozzle.
See Figure F.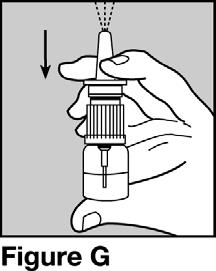
Step 6. Check to see if your Calcitonin Salmon Nasal Solution has been primed.
- To make sure you get the right dose of Calcitonin Salmon Nasal Solution medicine you must prime each new bottle and pump before you use it for the first time.
- If your pharmacist or healthcare provider puts the bottle and pump together for you, check to see if it has already been primed by pressing on the pump 1 time. See Figure G.
- If you see a full spray from the pump, the bottle and pump has already been primed for you.
- If you do not see a full spray, you must prime the bottle and pump.
Priming your Calcitonin Salmon Nasal Solution:
Step 7.
- Hold the bottle upright with your pointer finger and middle finger on the two side arms of the pump, and your thumb on the bottom of the bottle. Firmly press down on the arms of the pump and press down again if needed until you see a full spray of medicine. See Figure G.
- Now your Calcitonin Salmon Nasal Solution is ready for you to use.
- Do not prime the pump before you use it each day because this will waste your medicine.
Giving your Calcitonin Salmon Nasal Solution dose:
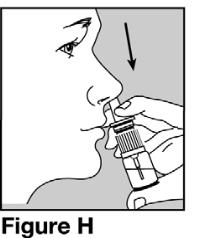
Step 8. Insert the nasal spray pump in 1 side of your nose.See Figure H.
- Keep your head upright. Carefully tilt the bottle and place the nose spray pump into 1 side of your nose.
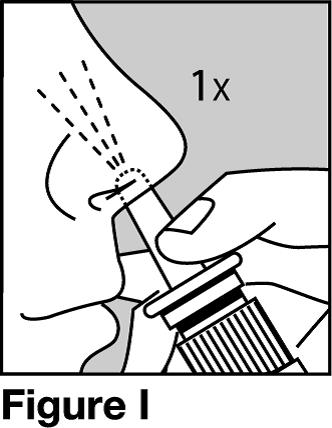
Step 9. Firmly press down on the nose spray pump to release the medicine. See Figure I.
- Give 1 spray of Calcitonin Salmon Nasal Solution, 1 time daily, in 1 side of your nose (nostril). Spray your medicine in a different side of your nose each day.
- You do not need to breathe in or inhale while you are giving your dose.
- You may not feel the spray inside your nose.
- Some of the medicine may drip out of your nose. This is normal, and you are still getting all of the medicine you need.
Cleaning your Calcitonin Salmon Nasal Solution pump:
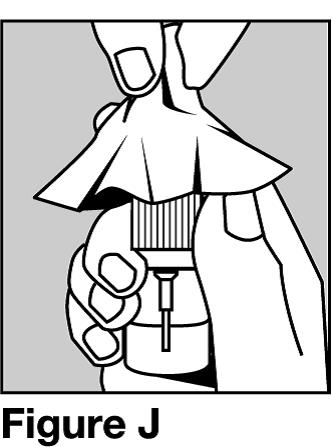
Step 10. Wipe the nose spray pump with a clean, damp cloth 1 to 2 times a week. See Figure J.
Dry the nose spray pump with a clean cloth.
Storing your Calcitonin Salmon Nasal Solution:
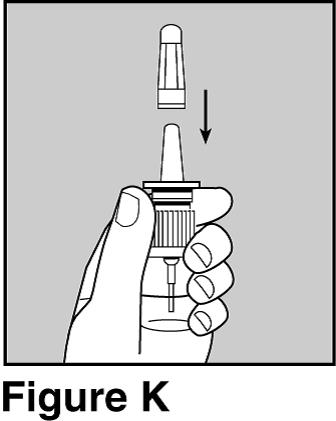
Step 11. Gently put the protective cap back on the nasal spray pump.
- Hold the bottle with 2 fingers under the two side arms of the pump. See Figure K.
- Be careful not push down on the pump while you are putting the cap back on it.
- Do not refrigerate Calcitonin Salmon Nasal Solution between doses.
- Store Calcitonin Salmon Nasal Solution upright.
- Do not shake the bottle.
When should I throw away Calcitonin Salmon Nasal Solution?
- Unopened, refrigerated bottles can be used until the expiration date stamped on the bottle and box.
- Throw away Calcitonin Salmon Nasal Solution after you use 30 doses (sprays).
- Throw away Calcitonin Salmon Nasal Solution bottles left at room temperature (opened or unopened) for more than 35 days.
For more information on Calcitonin Salmon Nasal Solution and how to put it together, call Par Pharmaceutical at 1-800-828-9393.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
- PRINCIPAL DISPLAY PANEL - CARTON
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALCITONIN SALMON
calcitonin salmon spray, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49884-161 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCITONIN SALMON (UNII: 7SFC6U2VI5) (CALCITONIN SALMON - UNII:7SFC6U2VI5) CALCITONIN SALMON 200 [iU] in 0.09 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) NITROGEN (UNII: N762921K75) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49884-161-11 3.8 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/08/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076979 06/08/2009 Labeler - Par Pharmaceutical, Inc. (092733690)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

