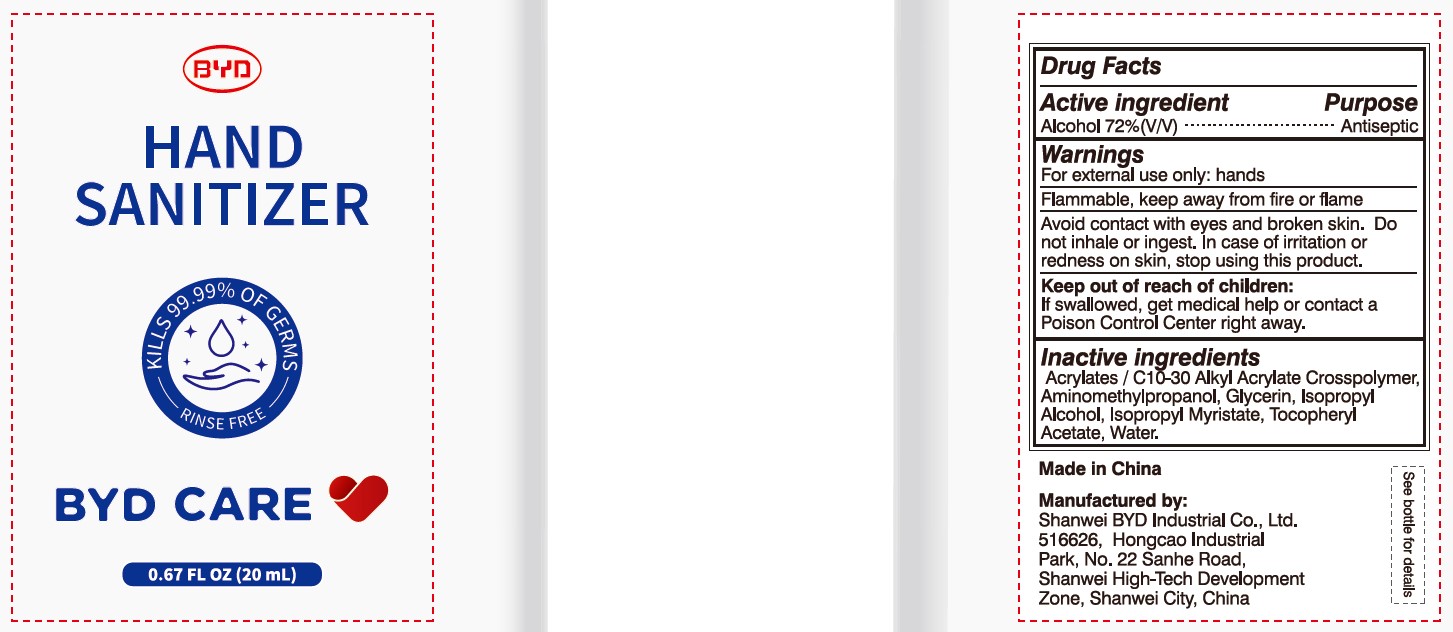
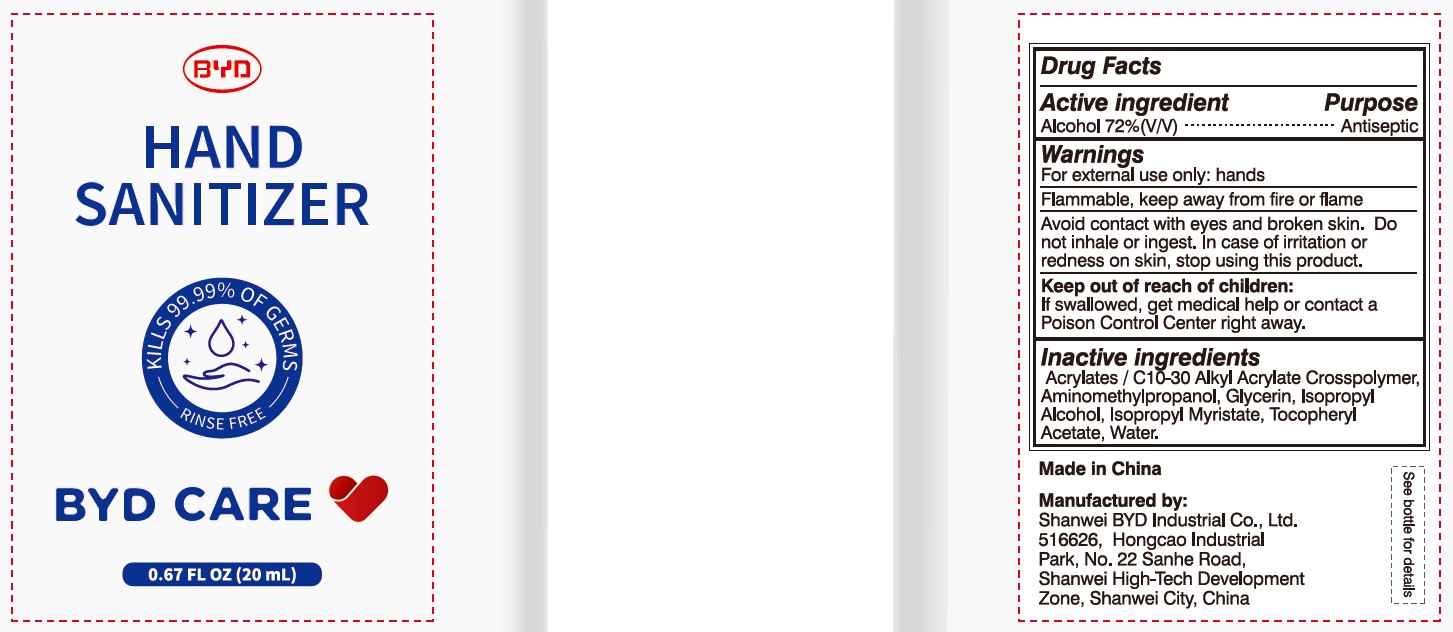
BYD CARE Hand Sanitizer XD-A04 by Shanwei BYD Industrial Co Ltd
BYD CARE Hand Sanitizer XD-A04 by
Drug Labeling and Warnings
BYD CARE Hand Sanitizer XD-A04 by is a Otc medication manufactured, distributed, or labeled by Shanwei BYD Industrial Co Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
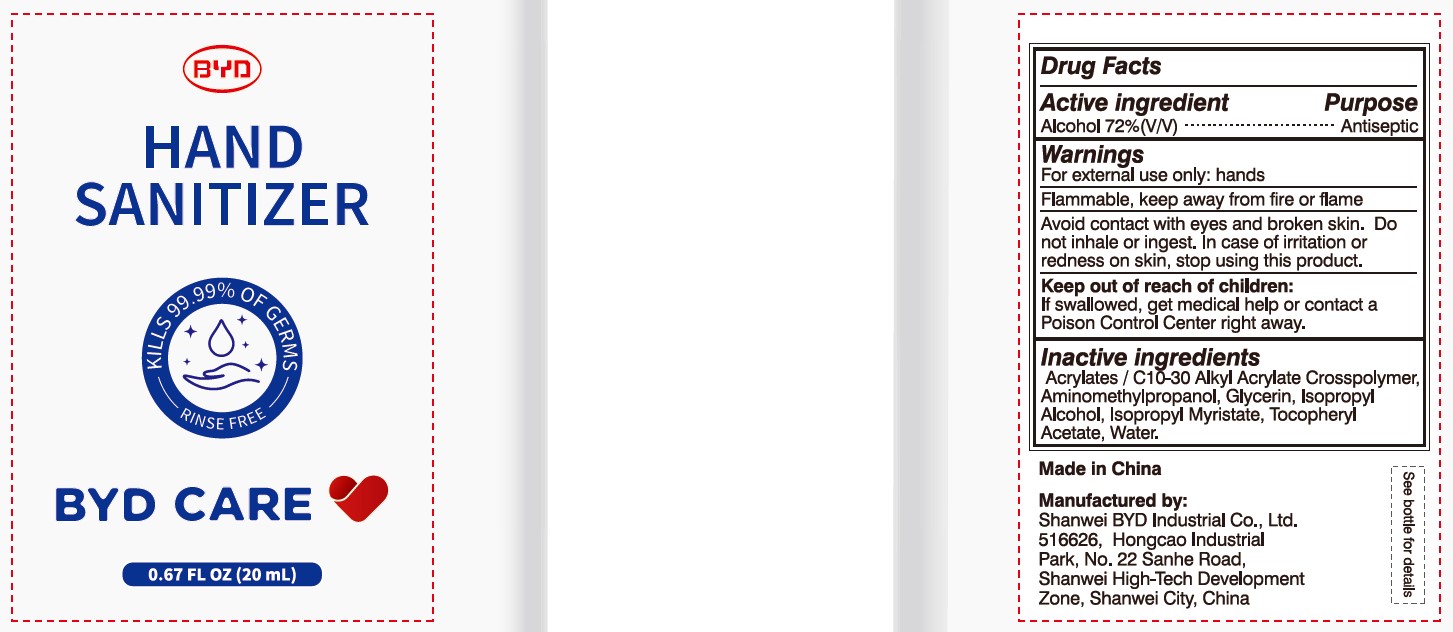
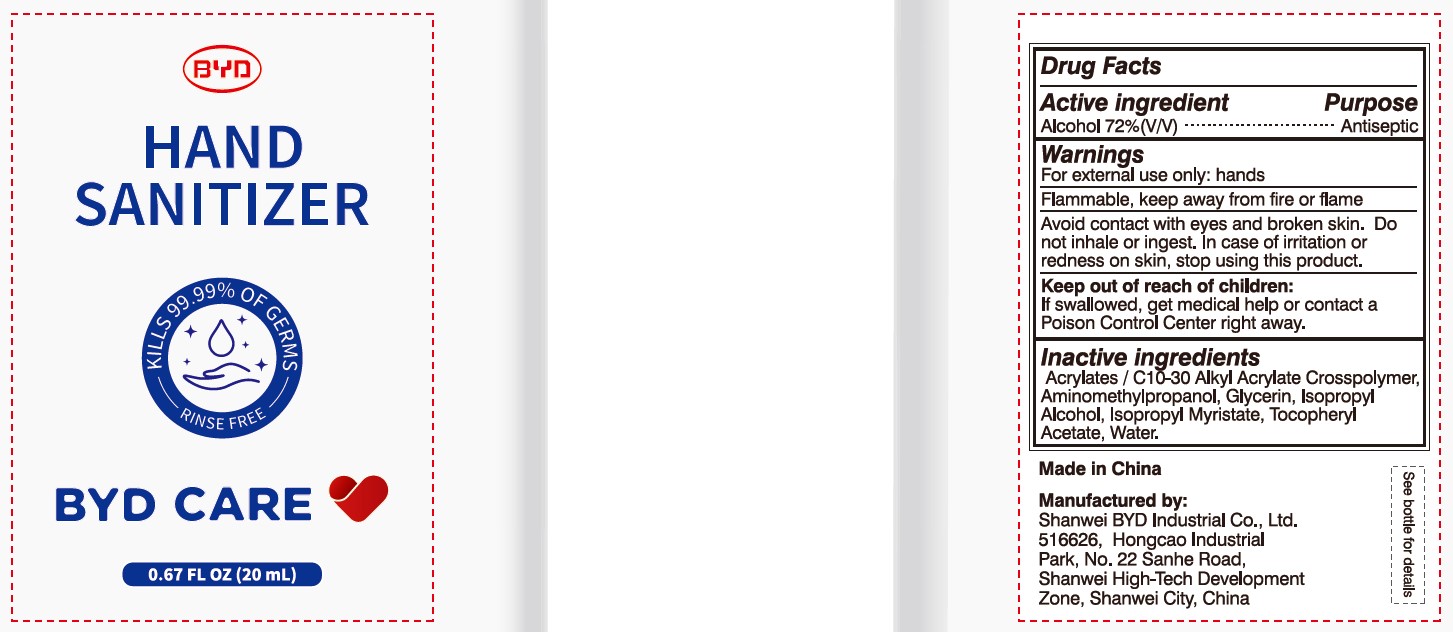
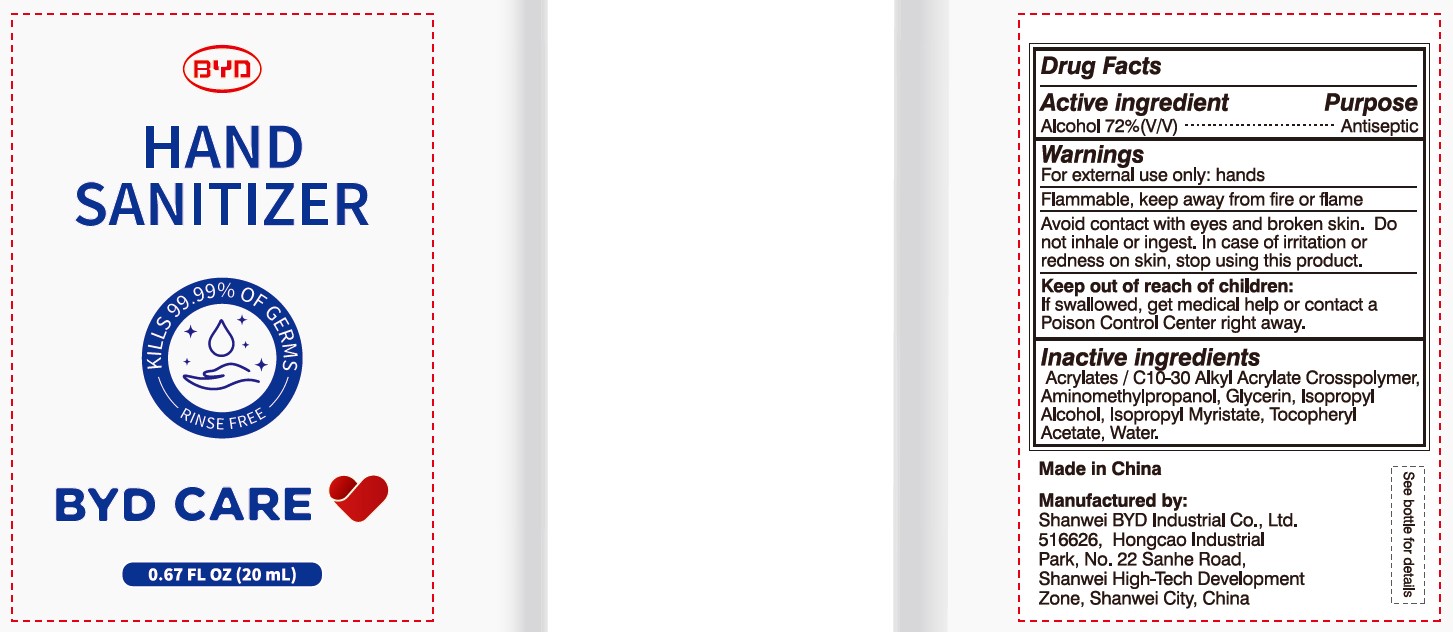
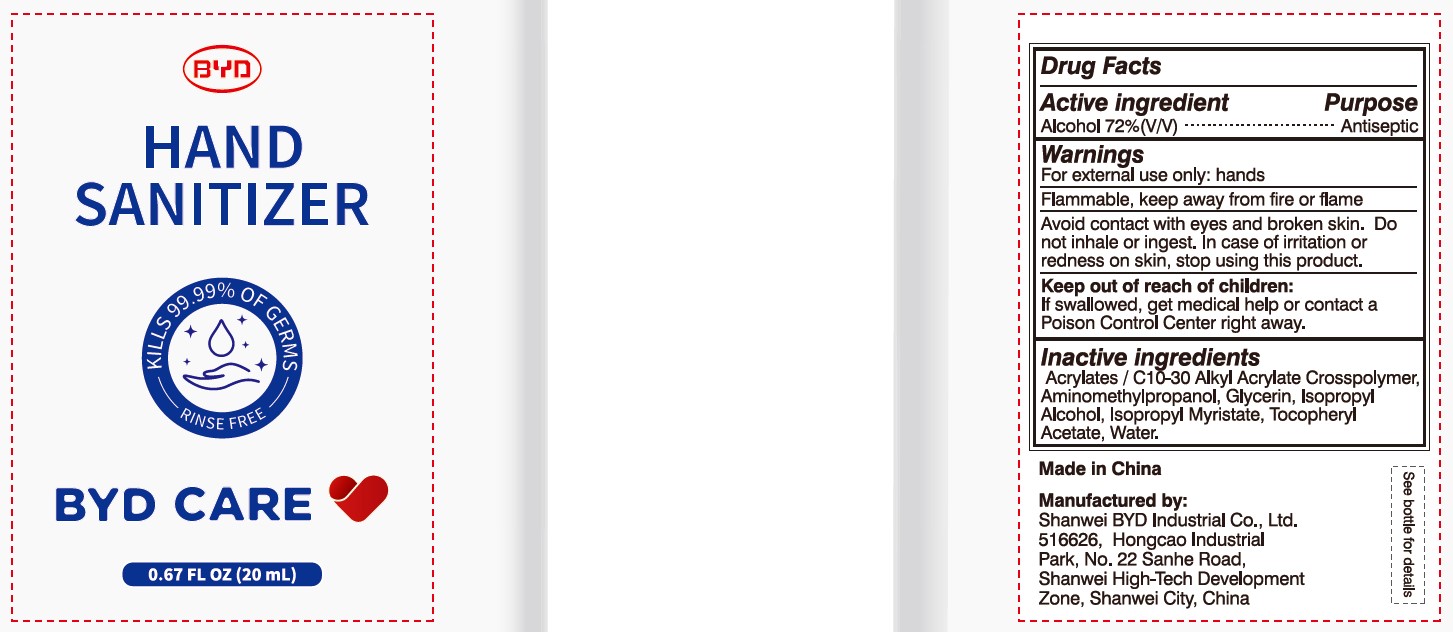
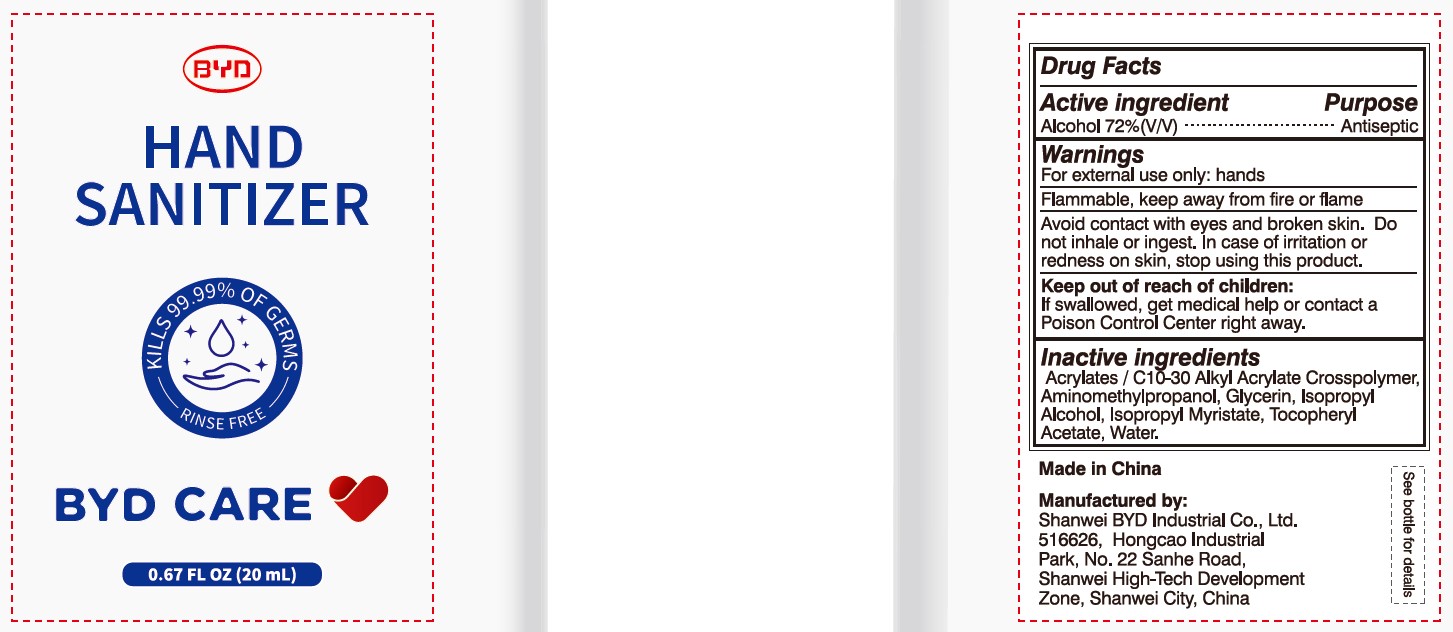
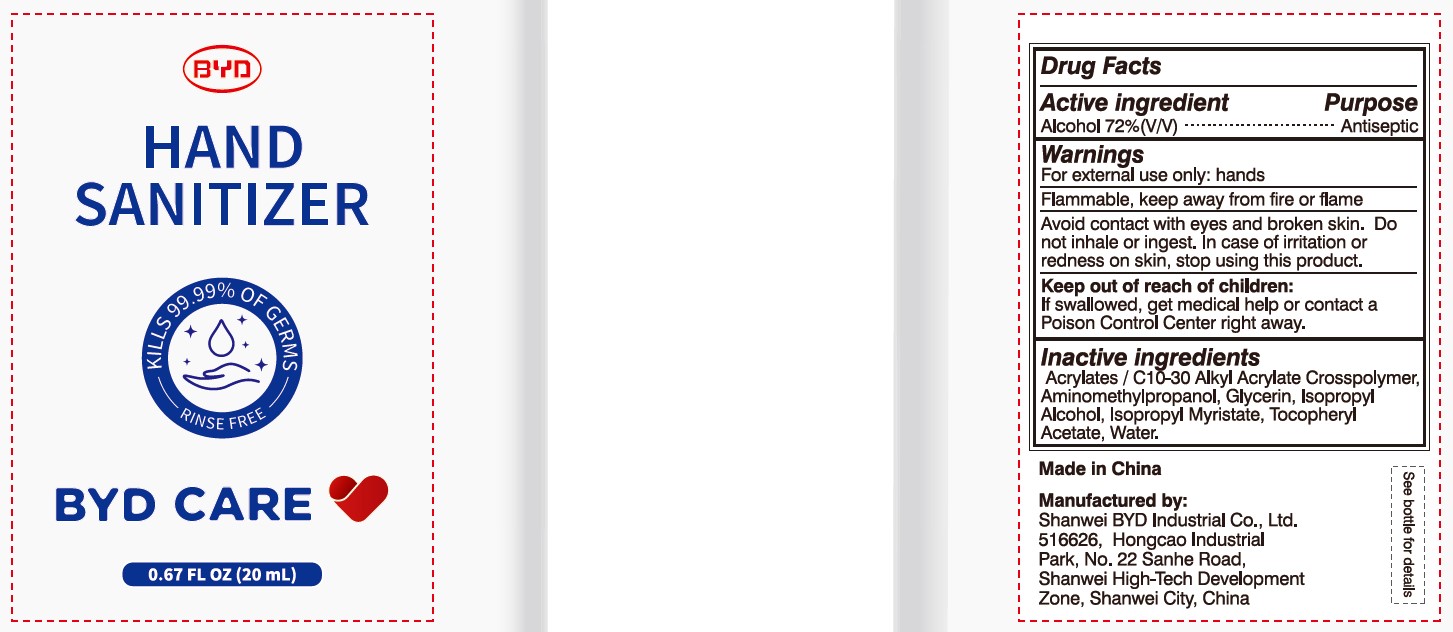
BYD CARE HAND SANITIZER XD-A04- alcohol gel
Shanwei BYD Industrial Co Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Misc.







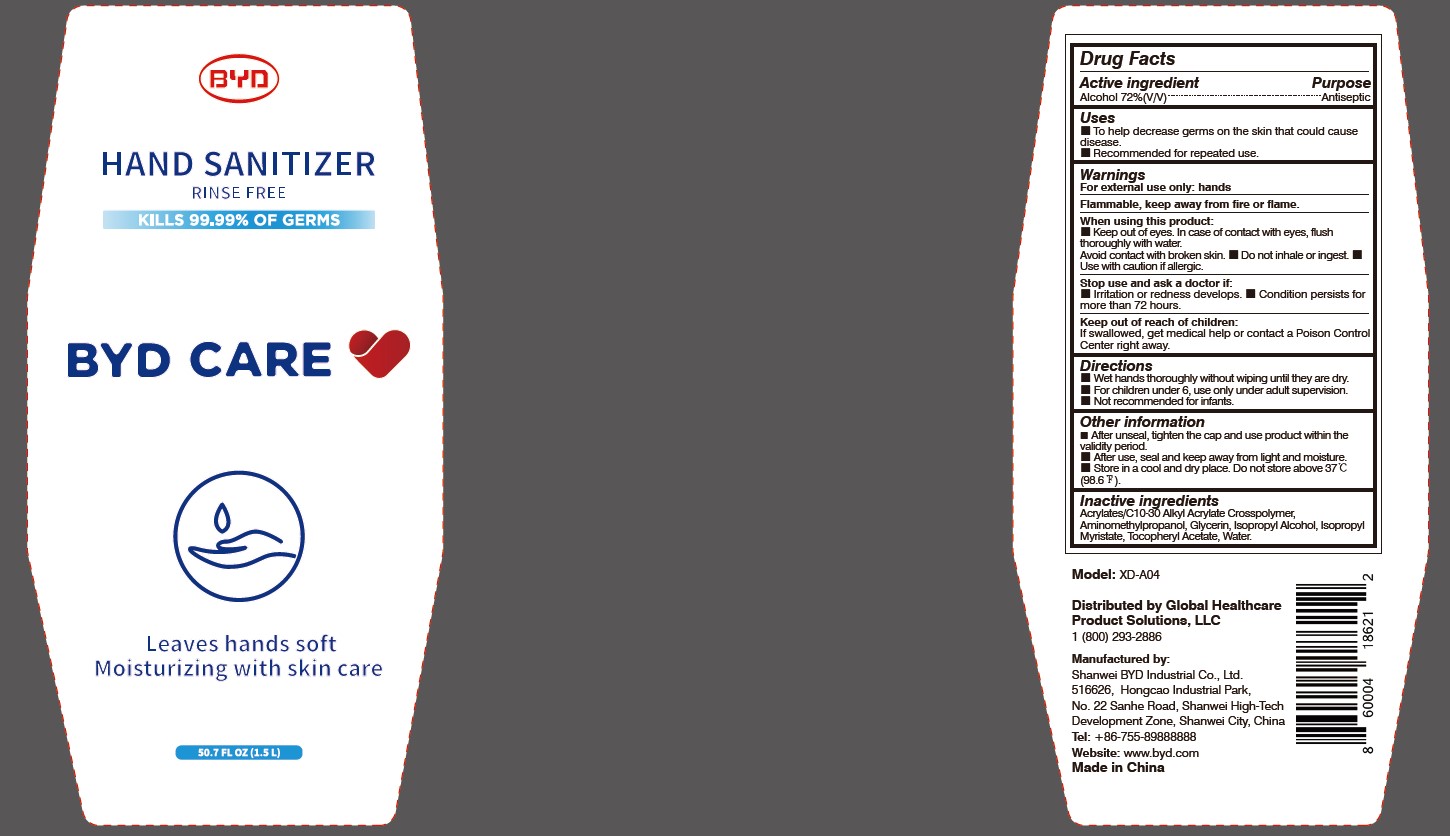
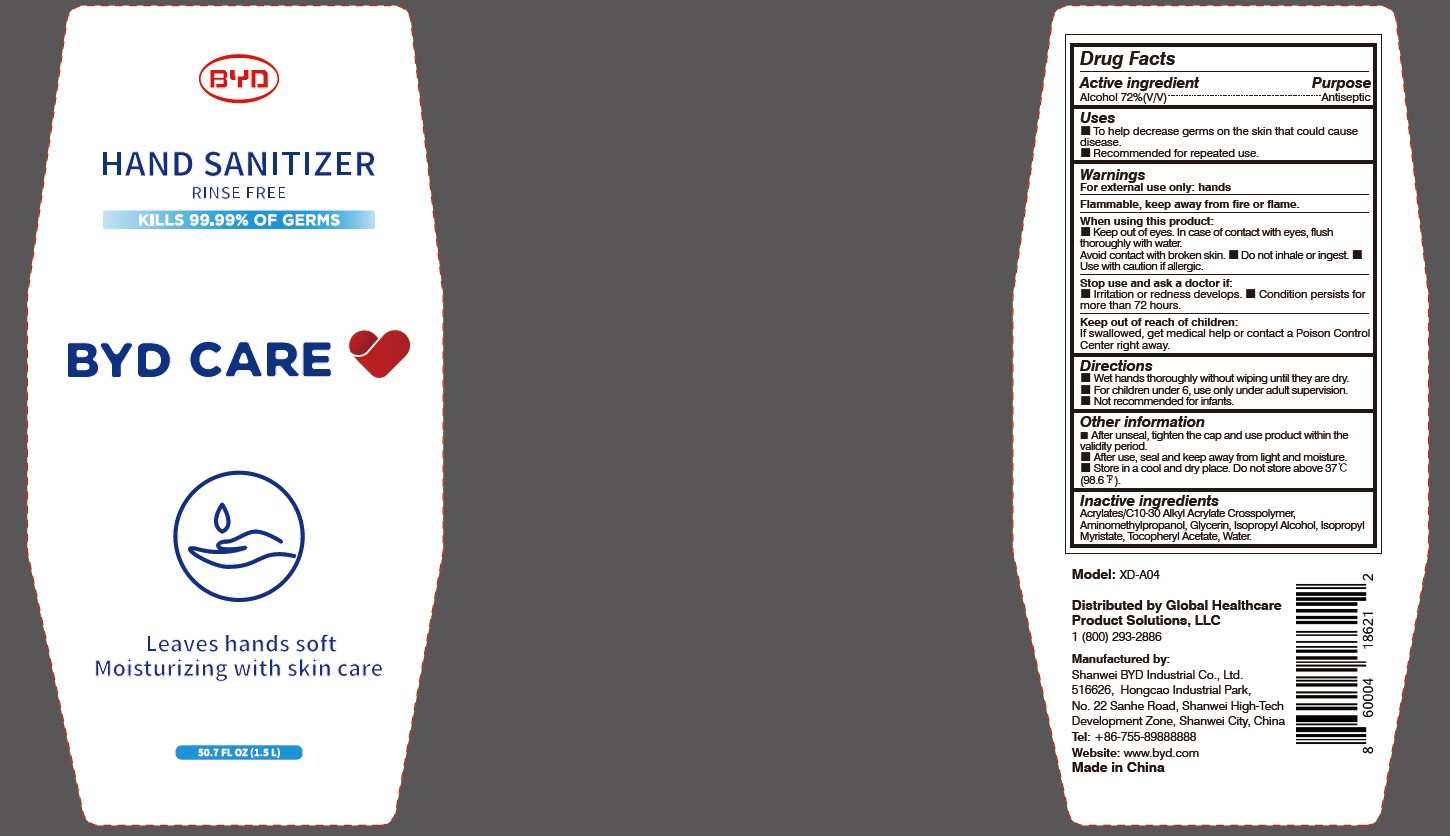
 Model: XD-A04
Model: XD-A04
Distributed by Global Healthcare Product Solutions, LLC
1 (800) 293-2886
Manufactured by:
Shanwei BYD Industrial Co., Ltd.
Shanwei High-Tech Development Zone, Shanwei, China
Tel: +86-755-89888888
Website: www.byd.com






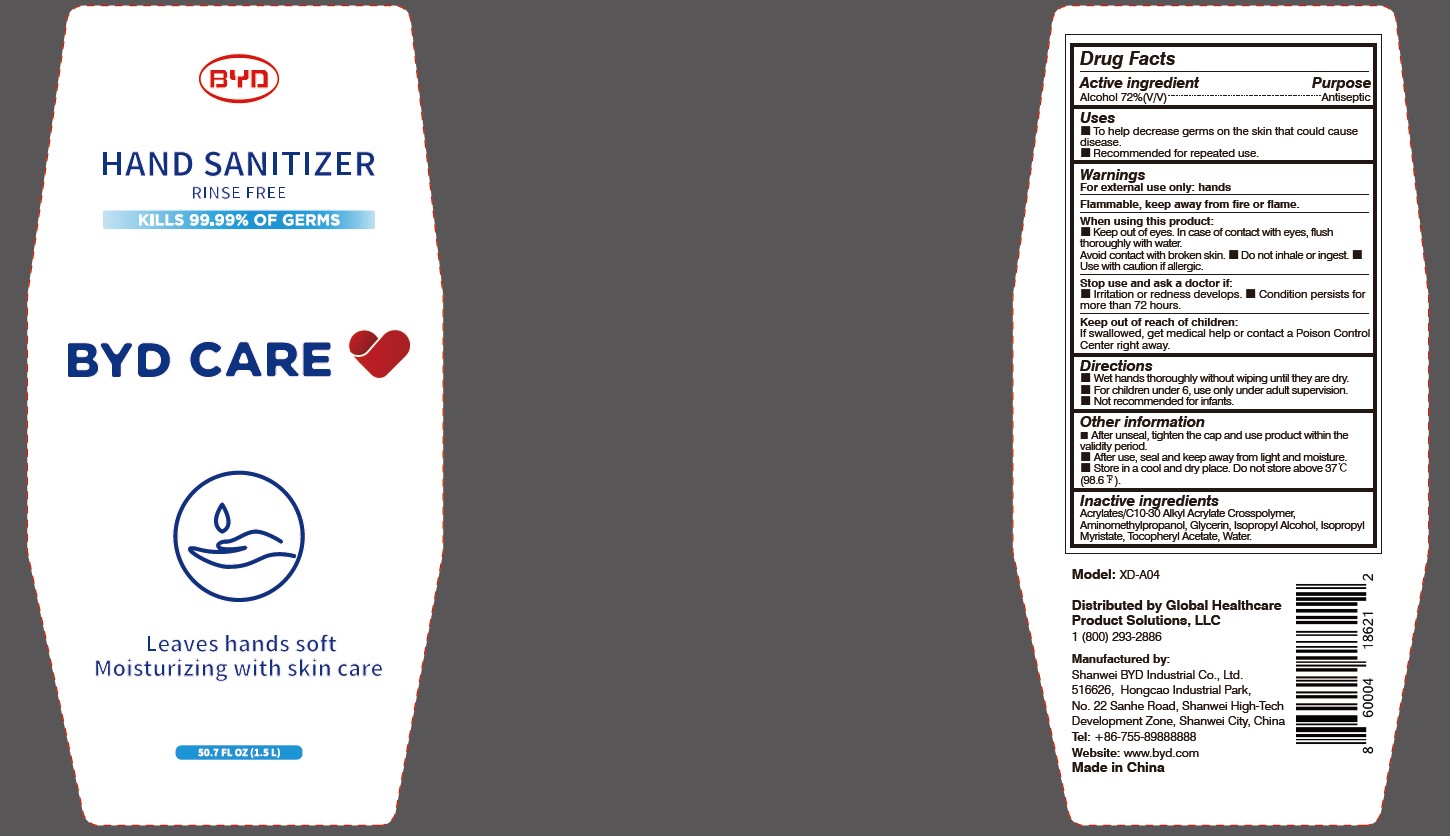
When using this product:







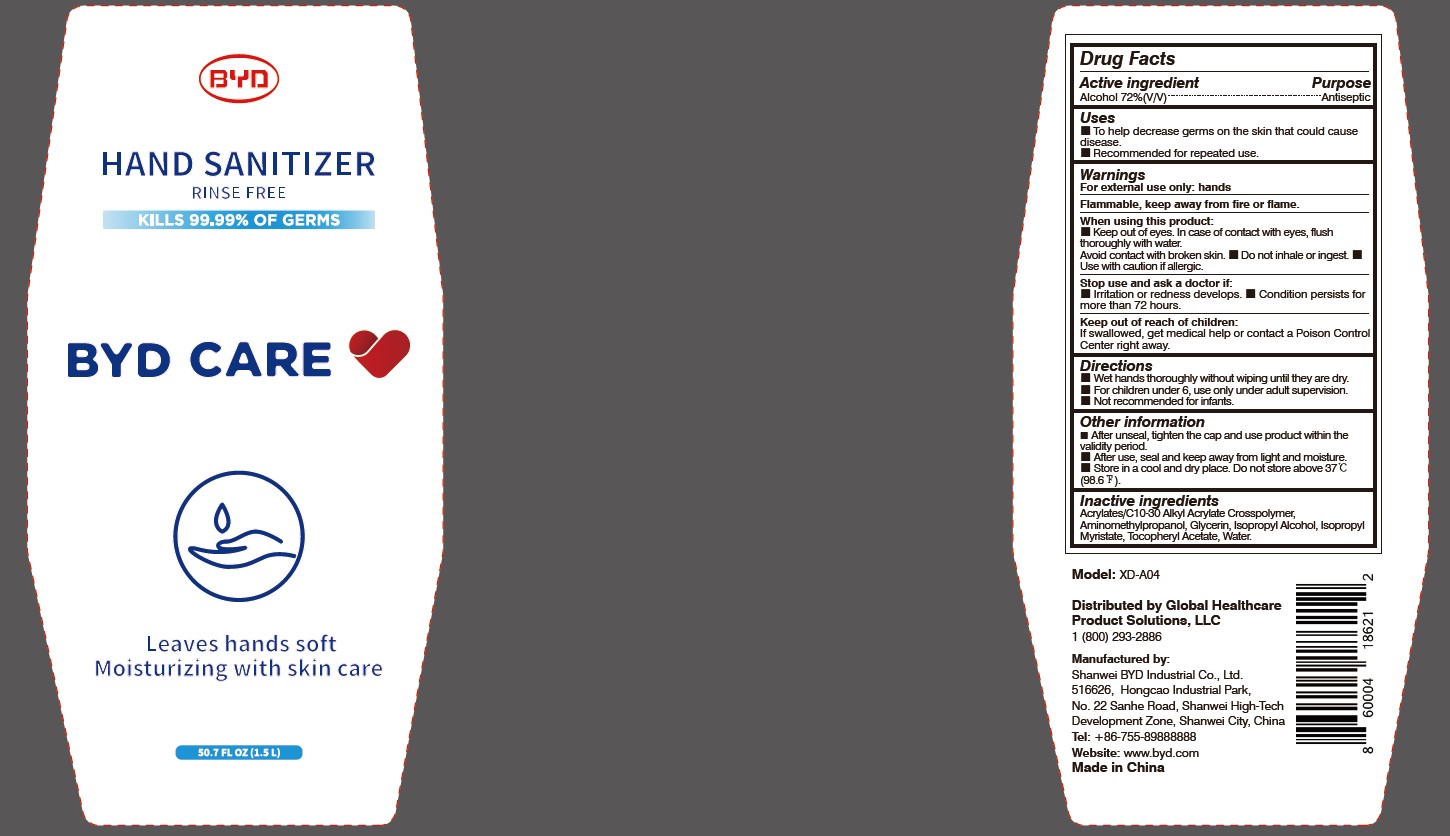 Keep out of eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin.
Keep out of eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin.
- Do not inhale or ingest.
- Use with caution if allergic.
When using this product:
-

 Keep out of eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin.
Keep out of eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin.
- Do not inhale or ingest.
- Use with caution if allergic.




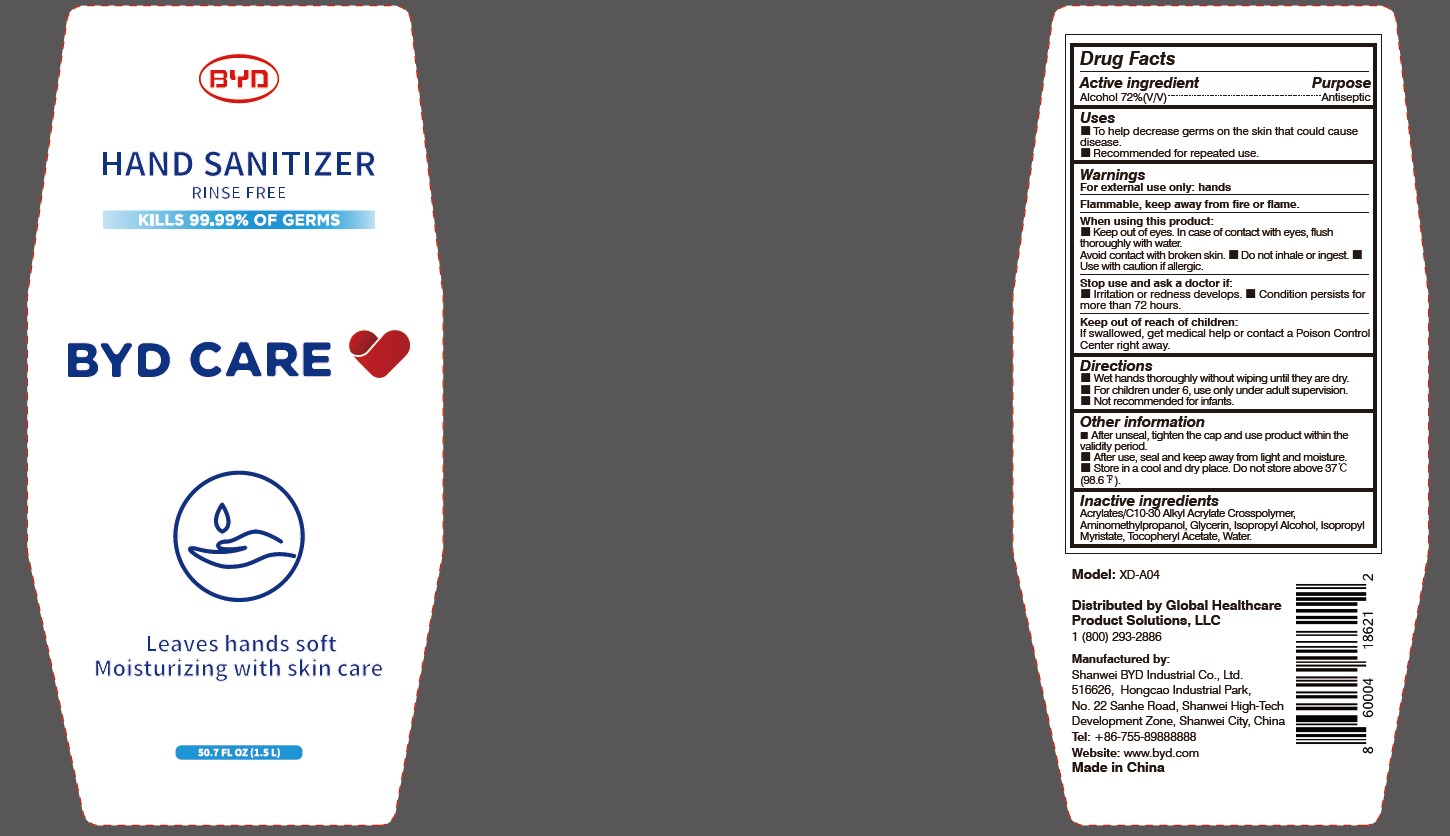
Stop use and ask a doctor if:
-






 Irritation or redness develops.
Irritation or redness develops.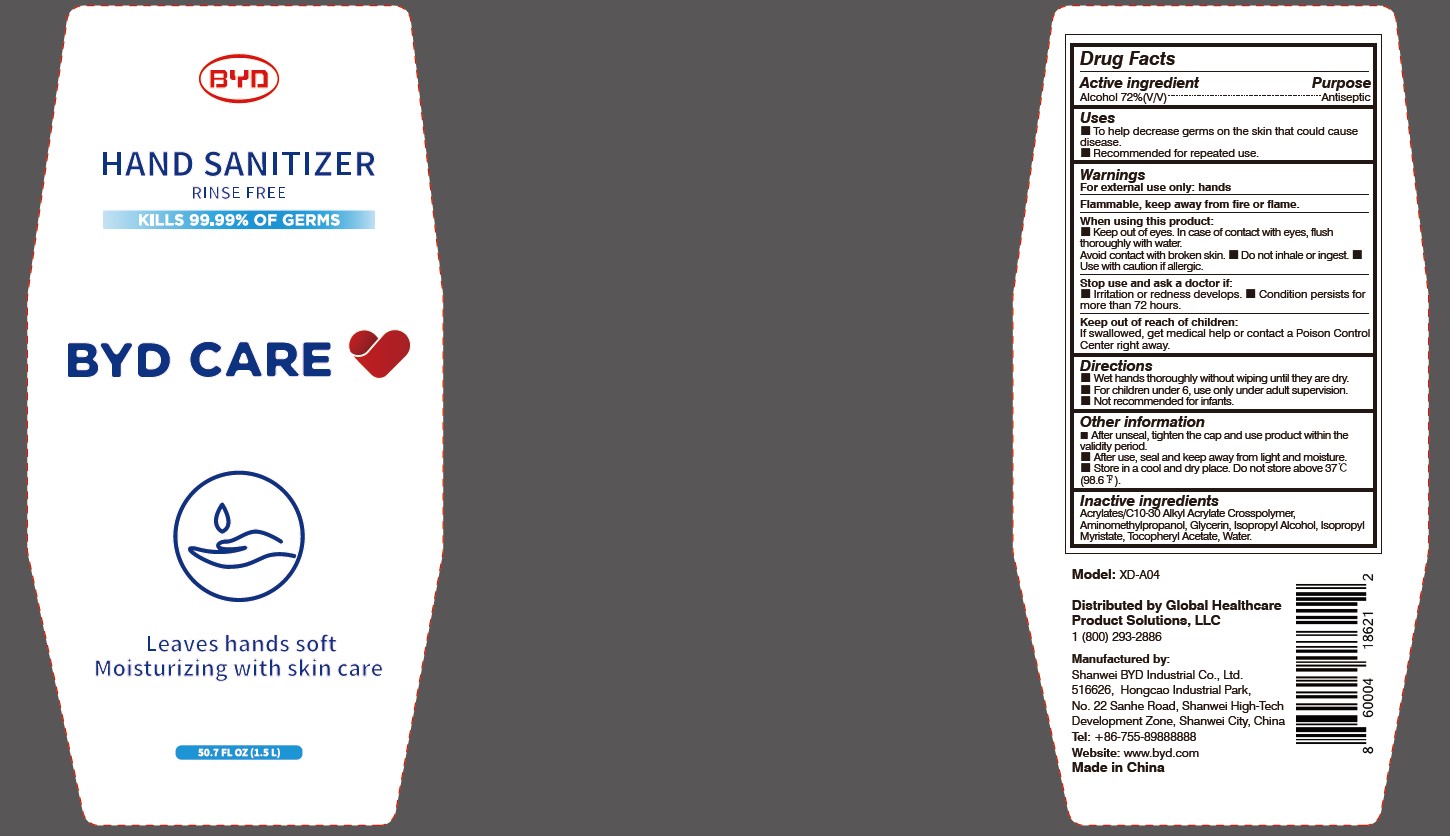
- Condition persists for more than 72 hours.
Keep out of reach of children:


 If swallowed, get medical help or contact a Poison Control Center right away.
If swallowed, get medical help or contact a Poison Control Center right away.




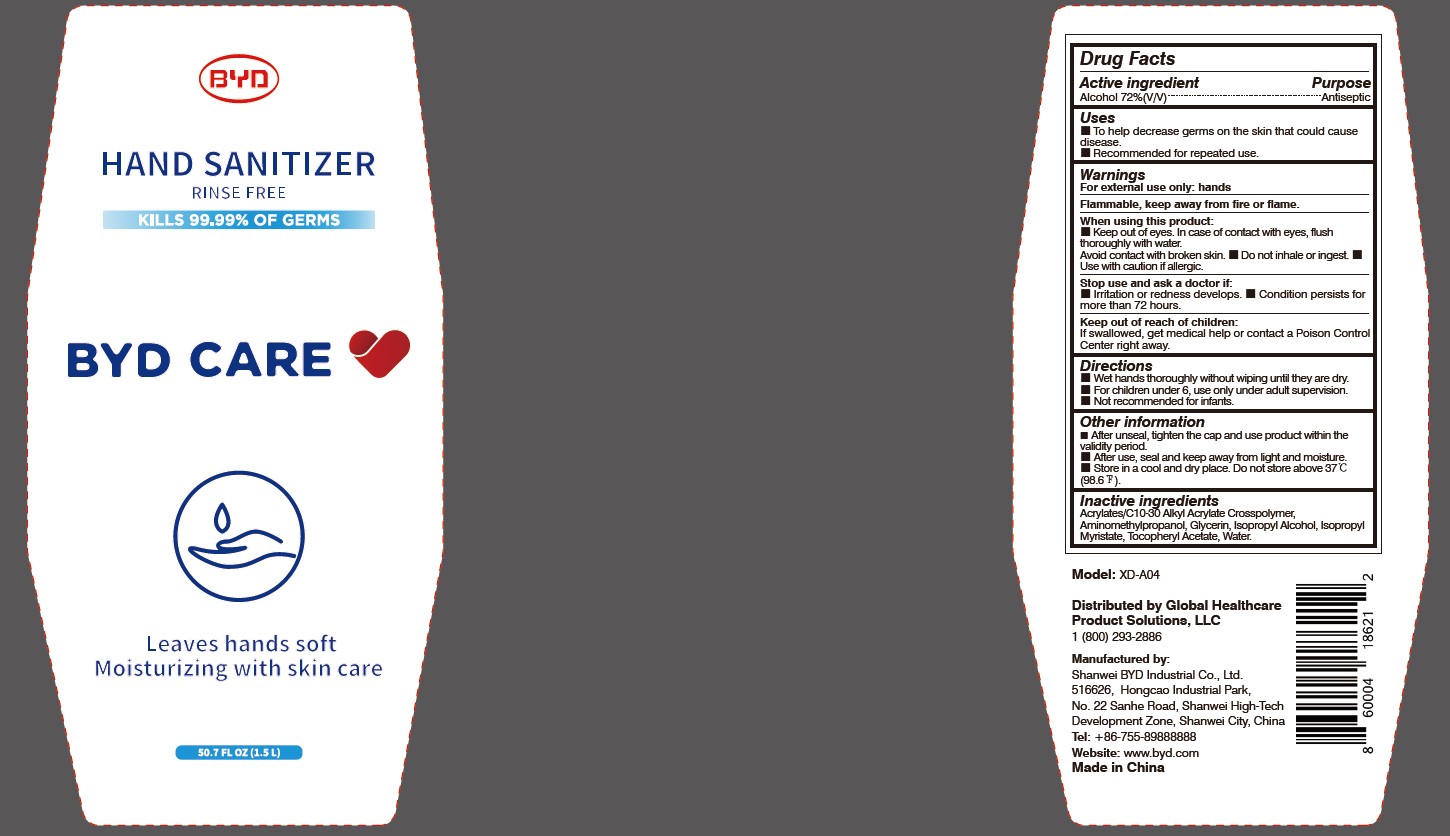
Directions:
-






 Wet hands thoroughly without wiping until they are dry.
Wet hands thoroughly without wiping until they are dry.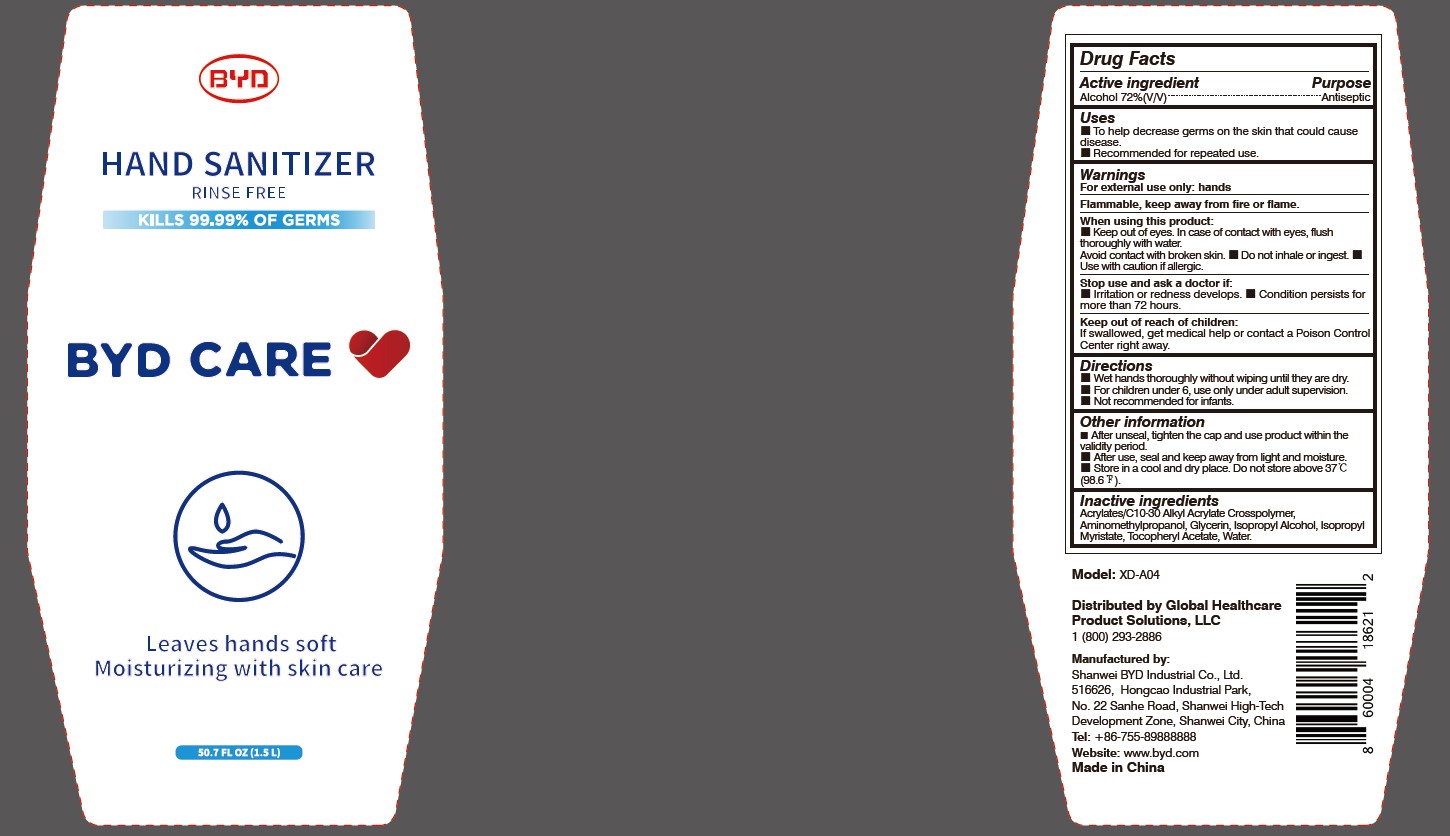
- For children under 6, use only under adult supervision.
- Not recommended for infants.
Other information:
-






 After unseal, tighten the cap and use product within the validity period.
After unseal, tighten the cap and use product within the validity period.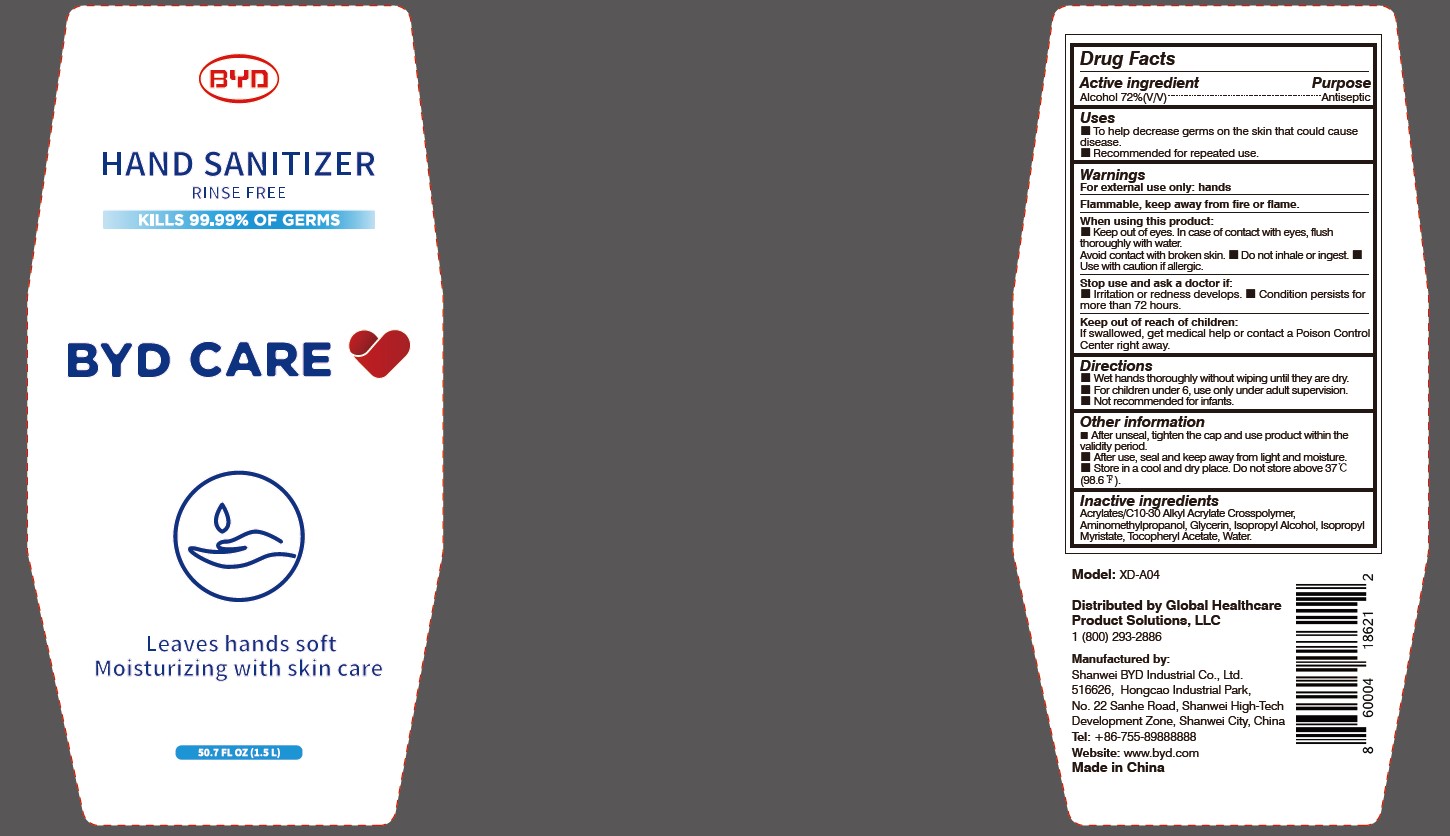
- After use, seal and keep away from light and moisture.
- Store in a cool and dry place. Do not store above 37 oC (98.6 oF).
| BYD CARE HAND SANITIZER XD-A04
alcohol gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Shanwei BYD Industrial Co Ltd (552102846) |
| Registrant - Shanwei BYD Industrial Co Ltd (552102846) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shanwei BYD Industrial Co Ltd | 552102846 | manufacture(73818-200) , label(73818-200) , pack(73818-200) | |


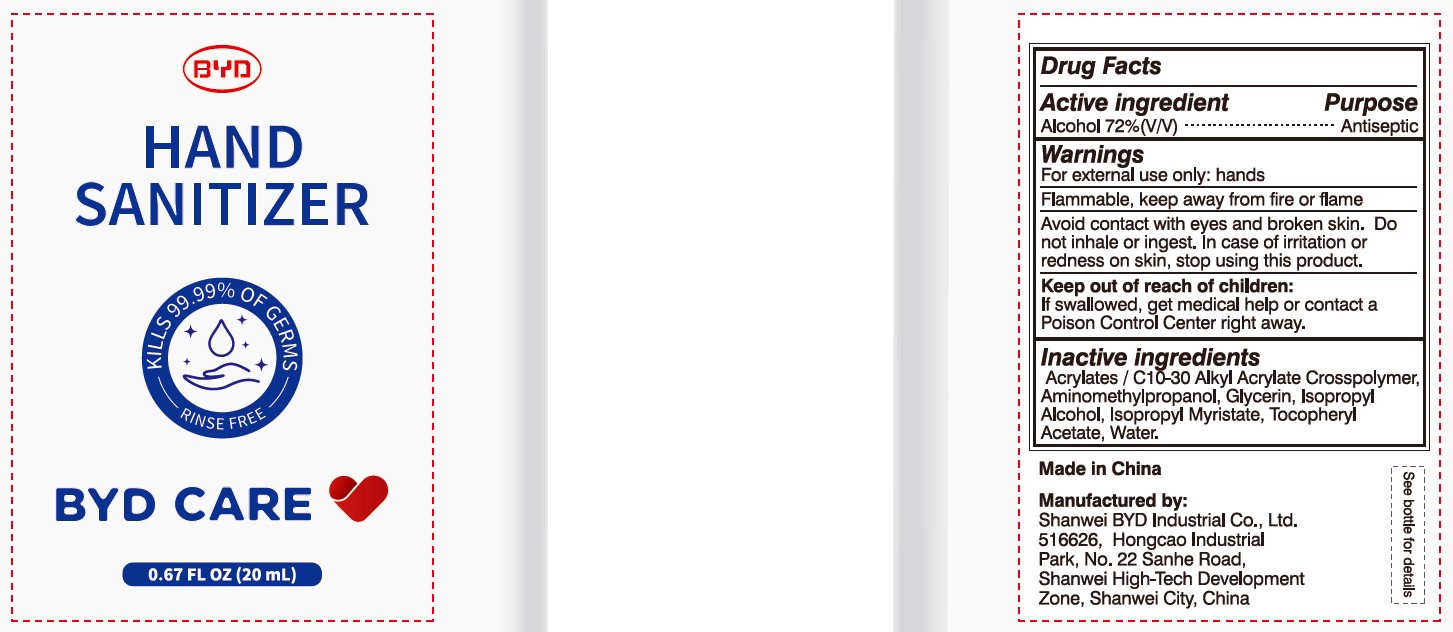
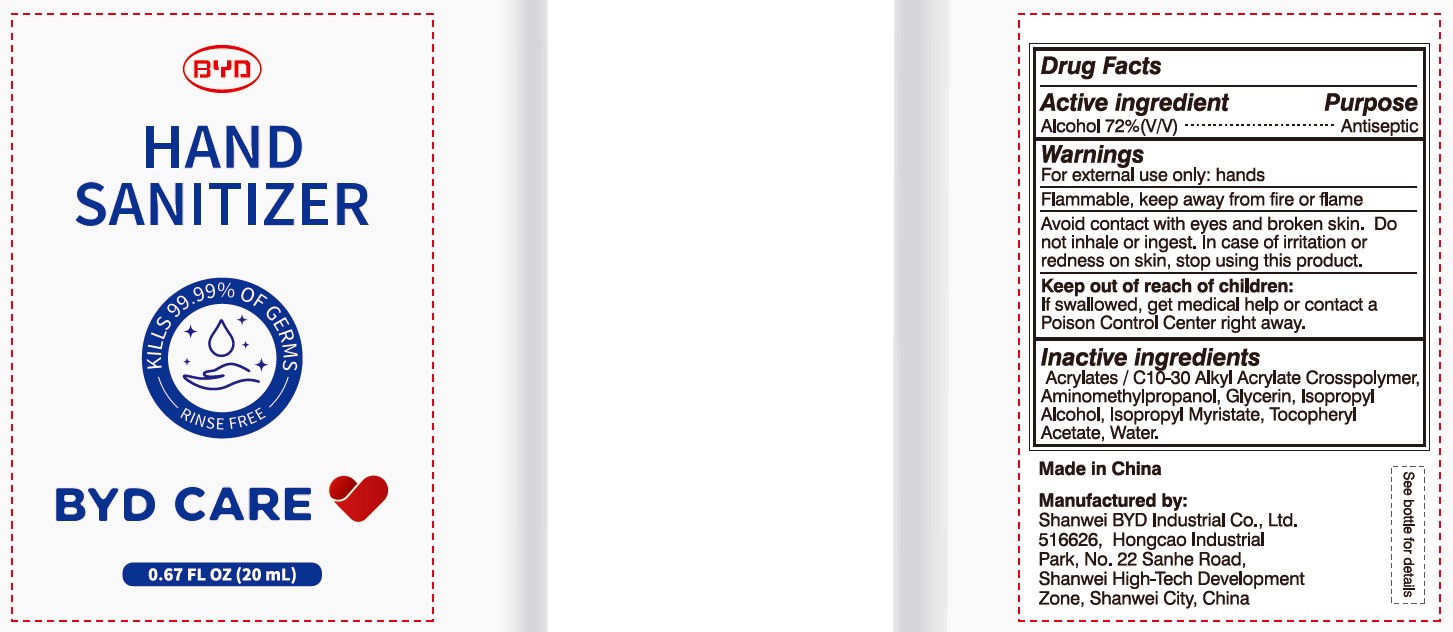
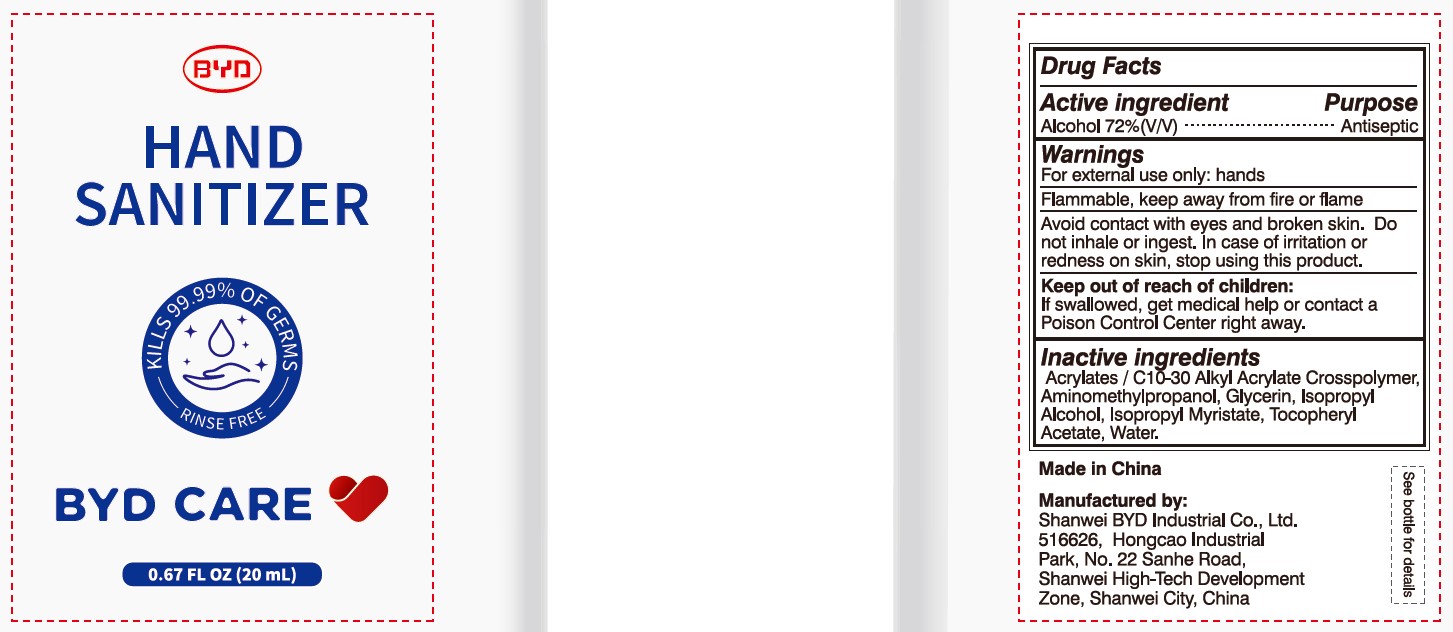
 Alcohol 72% (V/V)
Alcohol 72% (V/V)






 Antiseptic
Antiseptic




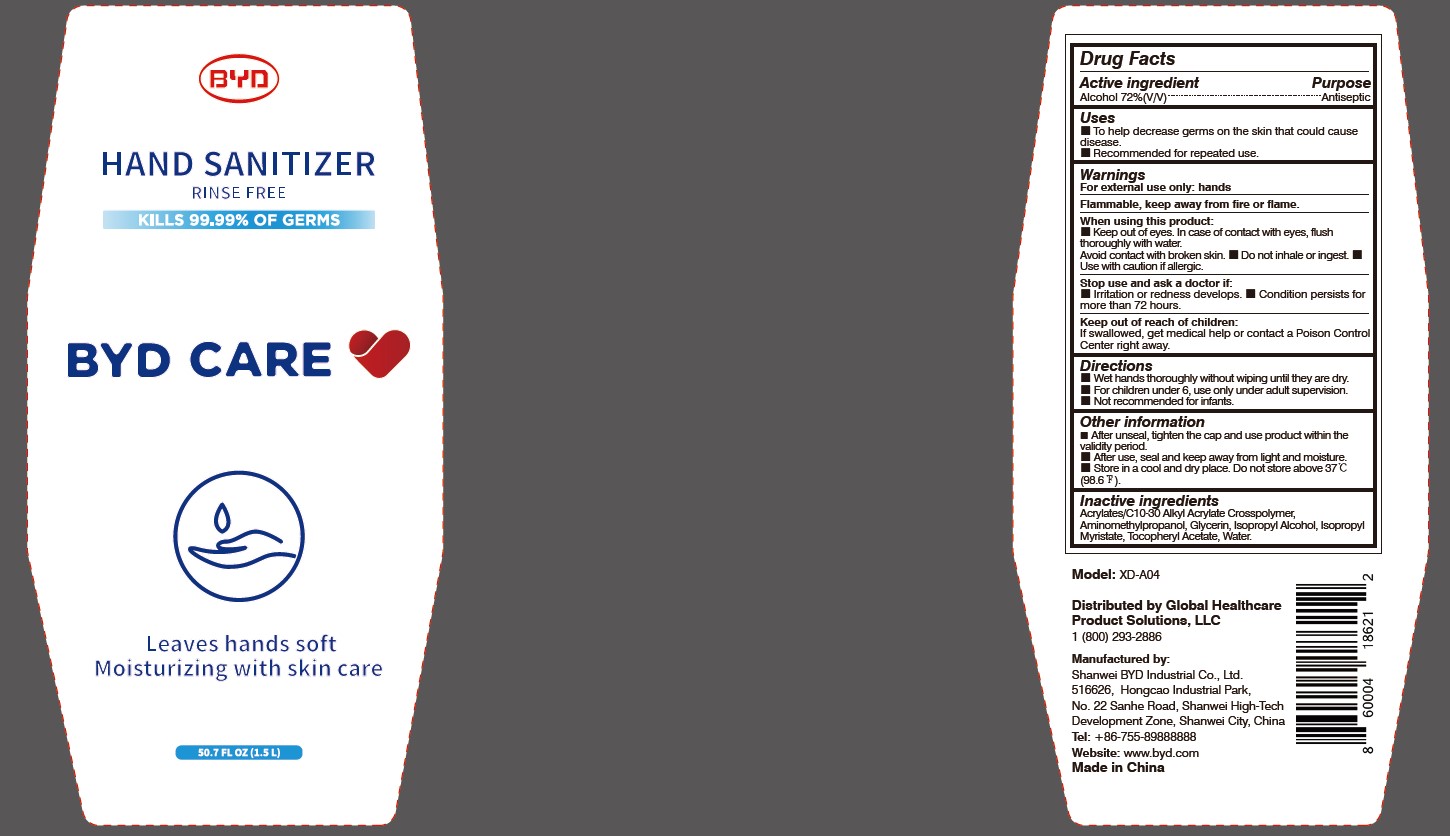







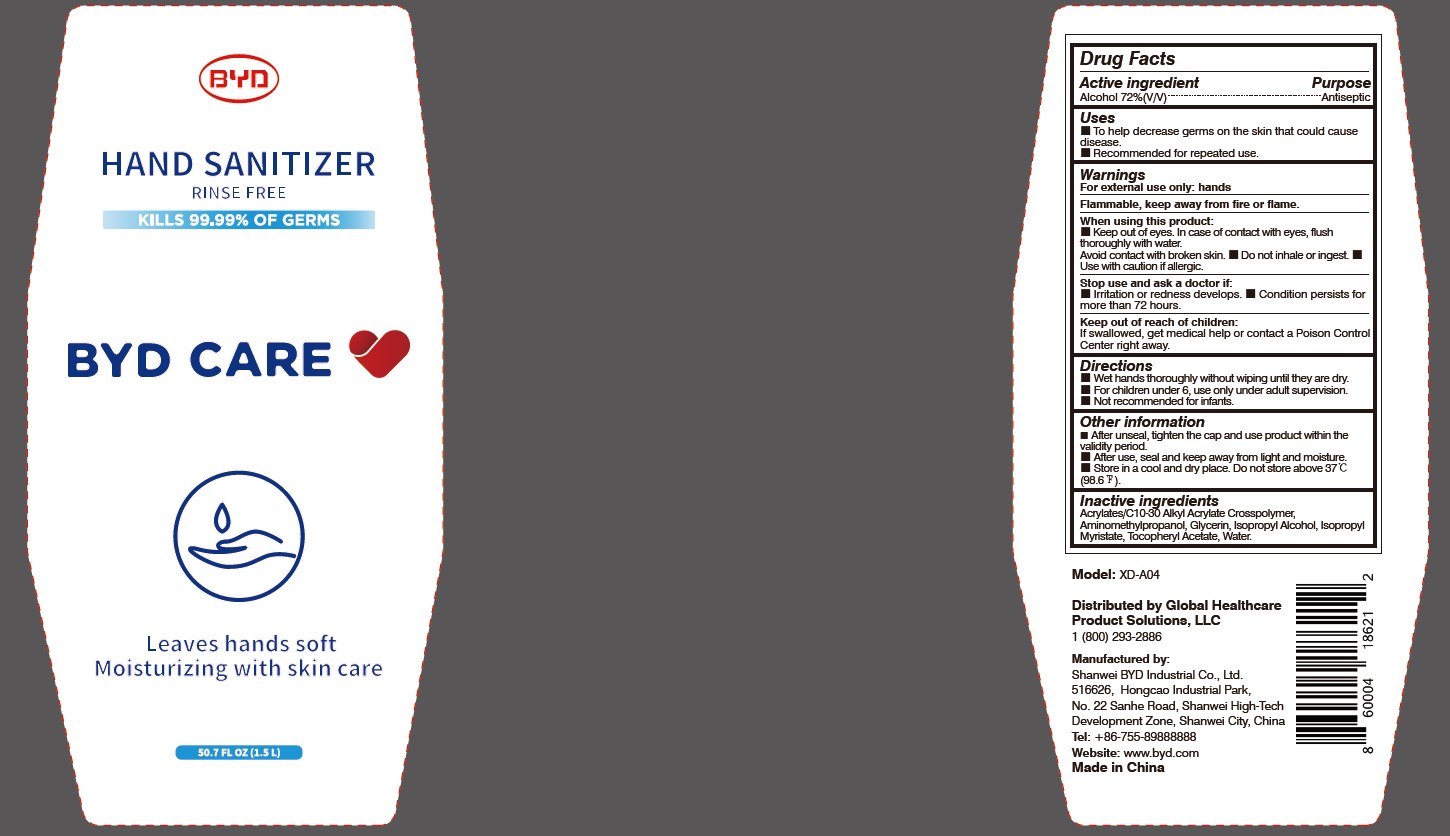 To Help decrease germs on the skin that could cause disease. Recommended for repeated use.
To Help decrease germs on the skin that could cause disease. Recommended for repeated use.


 For external use only: hands
For external use only: hands
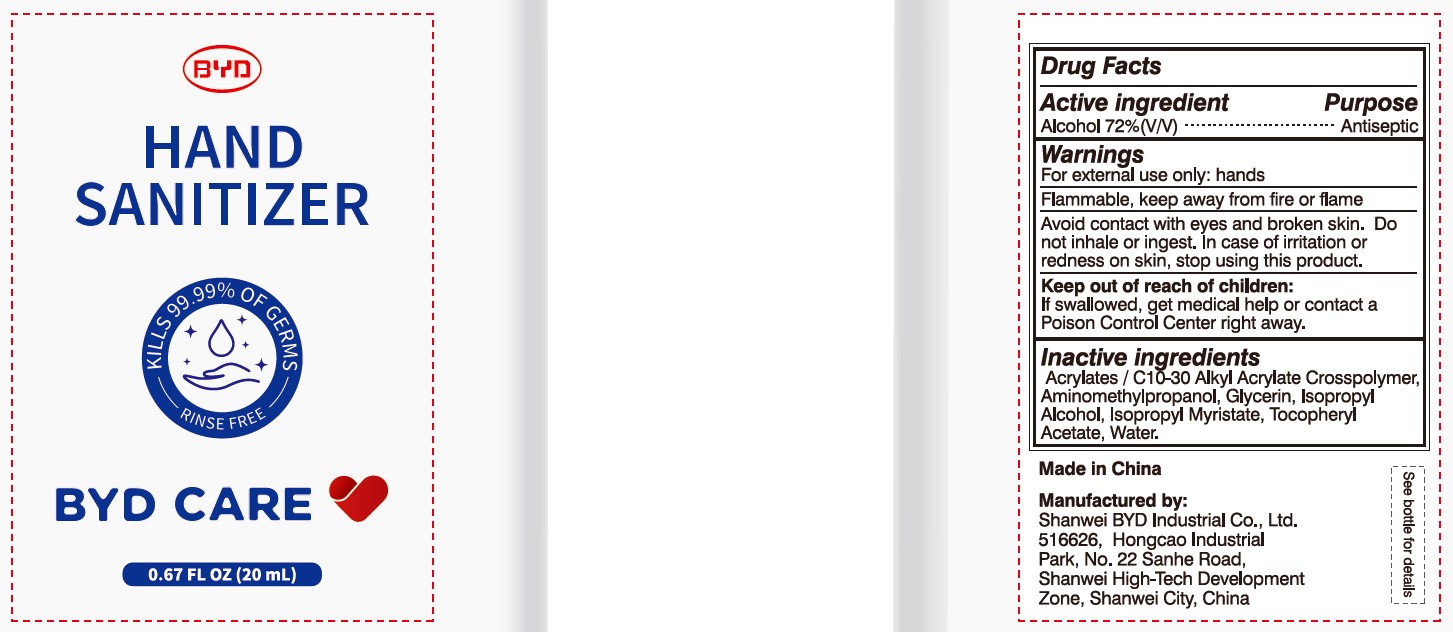




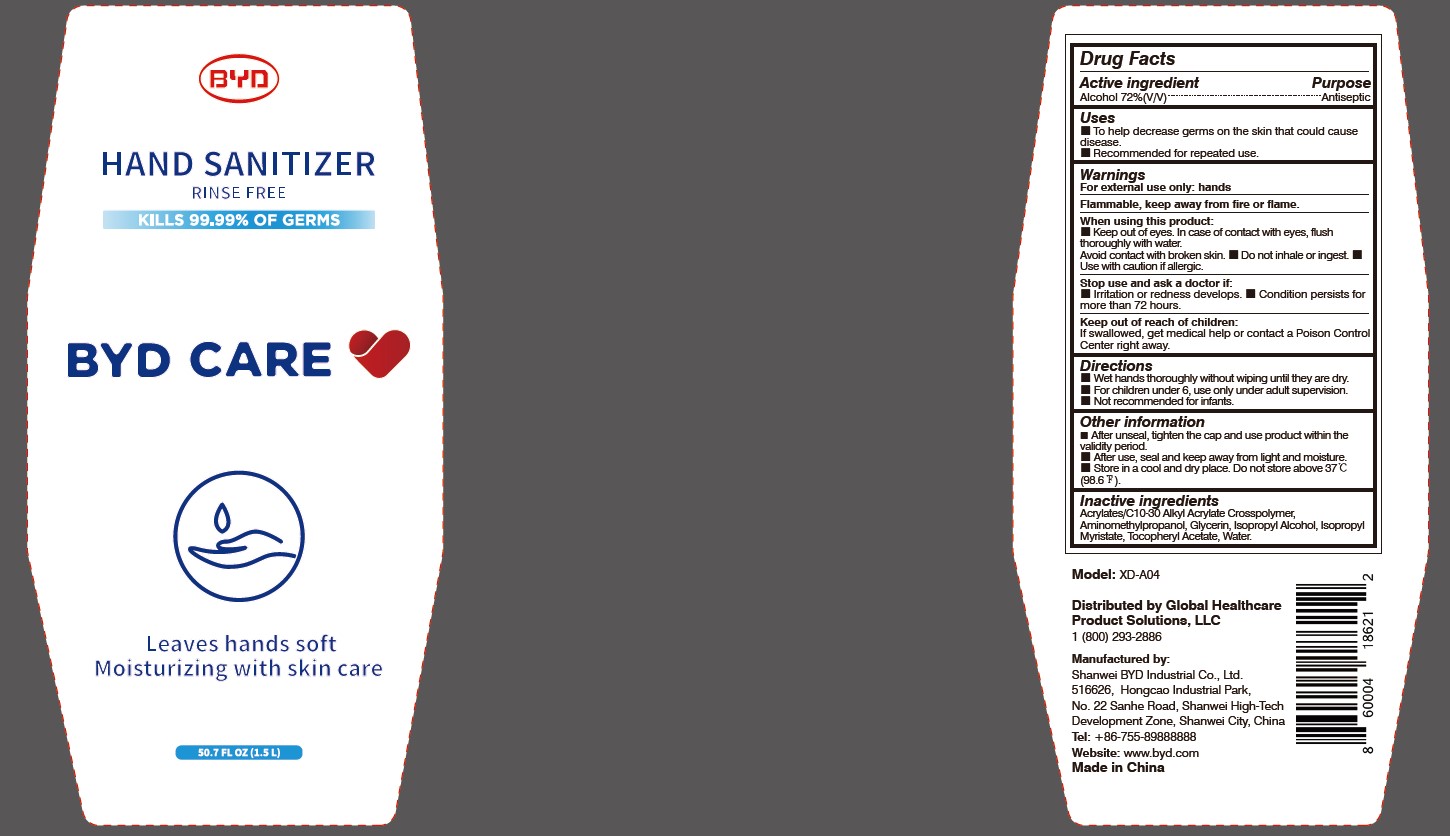



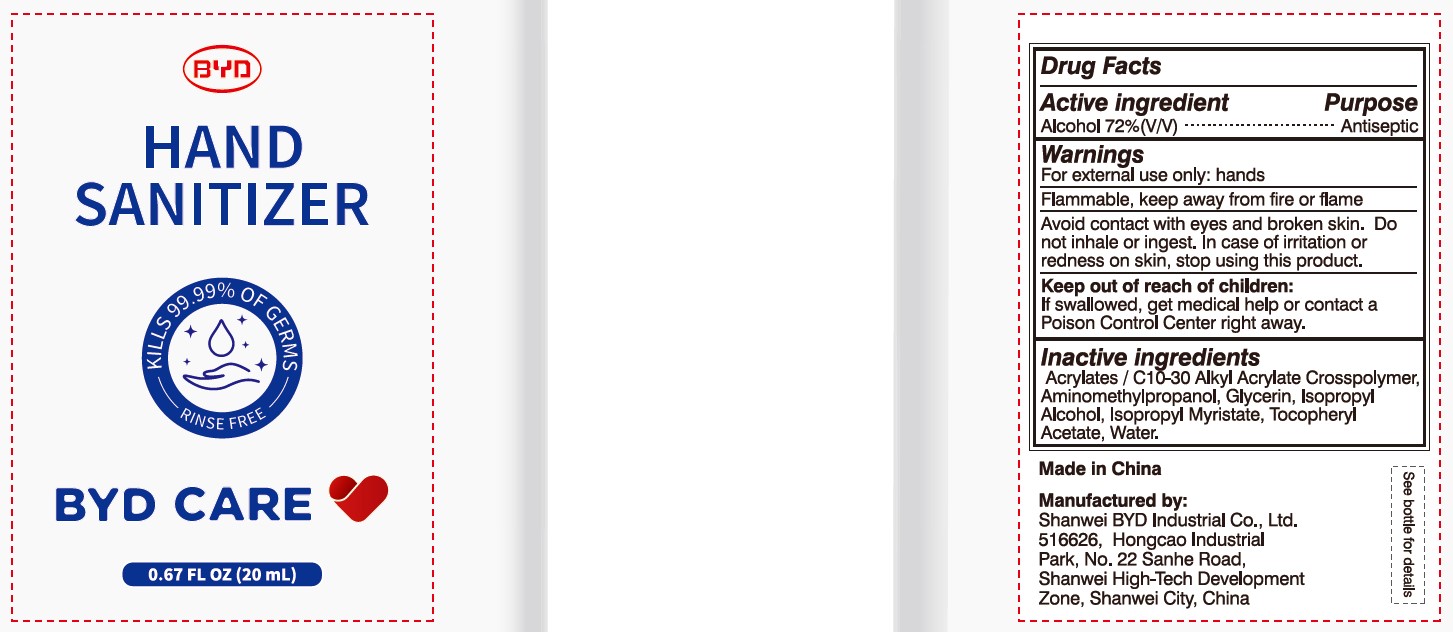




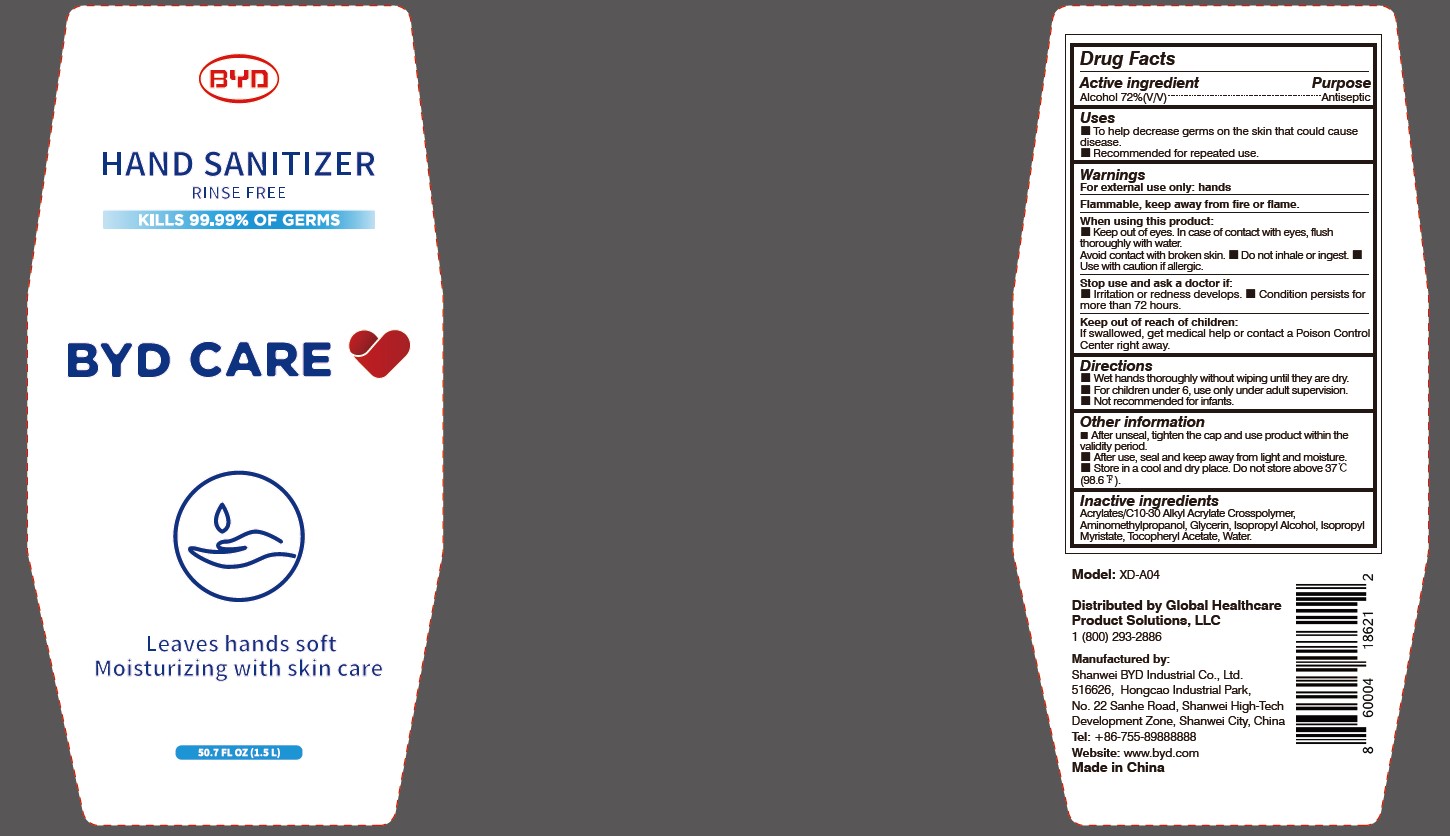 Arcylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethylpropanol, Glycerin, Isopropyl Alcohol, Isopropyl Myristate, Tocopheryl Acetate, Water
Arcylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethylpropanol, Glycerin, Isopropyl Alcohol, Isopropyl Myristate, Tocopheryl Acetate, Water