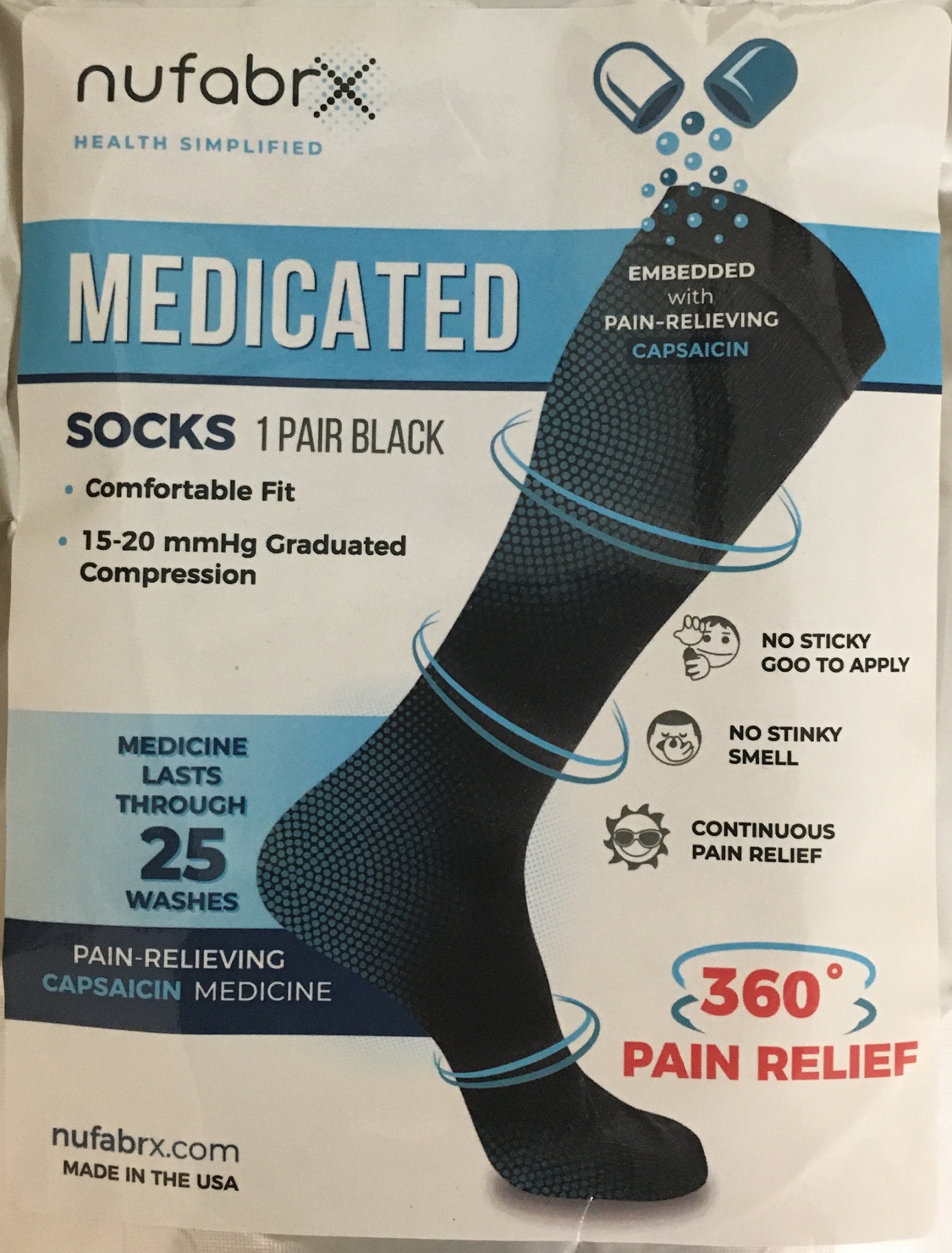
Nufabrx Grey Polka Dot Sock
Nufabrx Grey Polka Dot Sock by
Drug Labeling and Warnings
Nufabrx Grey Polka Dot Sock by is a Otc medication manufactured, distributed, or labeled by Textile-Based Delivery, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NUFABRX GREY POLKA DOT SOCK- capsaicin cloth
Textile-Based Delivery, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Nufabrx Grey Polka Dot Sock
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with: simple backache, arthritis, strains, bruises, and sprains.
Do not use
- Allergy Alert: if you are allergic to any ingredients of this product or chili peppers contact a doctor before use
- On wounds or to damaged skin
- With, or at same time as, other analgesic products
- With a heating pad
When using this product
- You may experience a burning sensation which is normal and related to the way the product works. With regular use, this sensation generally diminishes.
- Do not use immediately before or after activities such as swimming, showering, or sunbathing.
- Use only as directed.
- Avoid contact with eyes and mucous membranes or rashes.
- Wash hands after putting product on body and before touching your face or eyes.
Stop use and ask a doctor if
- condition worsens, symptoms persist more than 7 days or clear up and occur again within a few days
- rash, itching or excessive skin irritation develops
- If pregnant or breastfeeding consult a healthcare professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
| NUFABRX GREY POLKA DOT SOCK
capsaicin cloth |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Textile-Based Delivery, Inc. (079309716) |
Revised: 10/2021
Document Id: cecca646-64e8-10b3-e053-2a95a90a8647
Set id: a7219e85-e205-55f8-e053-2a95a90a45c9
Version: 2
Effective Time: 20211020