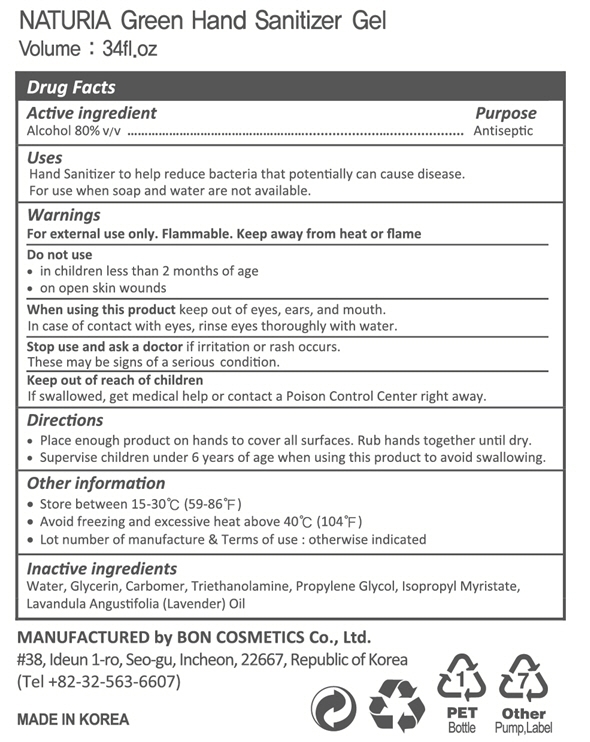
NATURIA GREEN HAND SANITIZER Gel by Bon Cosmetics. Co., Ltd Drug Facts
NATURIA GREEN HAND SANITIZER Gel by
Drug Labeling and Warnings
NATURIA GREEN HAND SANITIZER Gel by is a Otc medication manufactured, distributed, or labeled by Bon Cosmetics. Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NATURIA GREEN HAND SANITIZER GEL- alcohol gel
Bon Cosmetics. Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Water, Glycerin, Carbomer, Triethanolamine, Propylene Glycol, Isopropyl Myristate, Lavandula Angustifolia (Lavender) Oil
Do not use on the following body parts.
Around the eyes and ears, in the oral cavity, a wide range of body parts and damaged skin (may have irritating effects)
If the following symptoms appear, stop using them immediately and consult a doctor or pharmacist.
Symptoms of skin irritation
Other precautions
For external use only
Be careful not to get into your eyes, and if so, rinse well with clean water and consult a doctor or pharmacist.
Be careful not to inhale vapor when using it extensively or for a long time. (If you drink ethanol vapor in large quantities or repeatedly, irritation to the mucous membrane, headache, etc. may occur).
When repeated use on the same site, be careful as the skin may become rough due to degreasing.
When used in sealed bandages, cast bandages, packs, etc., irritation symptoms may appear.
Do not use for any other purpose.
| NATURIA GREEN HAND SANITIZER GEL
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bon Cosmetics. Co., Ltd (688293956) |
| Registrant - Bon Cosmetics. Co., Ltd (688293956) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bon Cosmetics. Co., Ltd | 688293956 | manufacture(74001-0007) | |